Abstract
Caloric restriction (CR) has long been known to increase median and maximal lifespans and to decreases mortality and morbidity in short-lived animal models, likely by altering fundamental biological processes that regulate aging and longevity. In rodents, CR was reported to delay the aging of the immune system (immune senescence), which is believed to be largely responsible for a dramatic increase in age-related susceptibility to infectious diseases. However, it is unclear whether CR can exert similar effects in long-lived organisms. Previous studies involving 2- to 4-year CR treatment of long-lived primates failed to find a CR effect or reported effects on the immune system opposite to those seen in CR-treated rodents. Here we show that long-term CR delays the adverse effects of aging on nonhuman primate T cells. CR effected a marked improvement in the maintenance and/or production of naïve T cells and the consequent preservation of T cell receptor repertoire diversity. Furthermore, CR also improved T cell function and reduced production of inflammatory cytokines by memory T cells. Our results provide evidence that CR can delay immune senescence in nonhuman primates, potentially contributing to an extended lifespan by reducing susceptibility to infectious disease.
Keywords: aging, immunity, T cell subsets, nutrition
Given the demographic trend in industrialized societies toward a marked increase in the elderly population, an intensive search is under way for interventions to reduce or delay the occurrence, and lessen the severity, of age-related disease. Among animal models of aging, the most promising intervention thus far is caloric restriction (CR), which has long been known to extend both median and maximal lifespans in rodents (1). CR similarly increases lifespan and retards the onset of age-related dysfunction in other short-lived organisms, including yeast, worms, flies, zebra fish, and spiders (as reviewed in refs. 2 and 3).
Unlike many interventions that increase average life span by reducing the negative effects of a specific age-related disease or of individual organ-specific aspects of aging, CR is believed to alter fundamental biological processes that regulate aging and longevity (4). However, its mechanisms remain unclear and are under intense study (5, 6). There is evidence in short-lived organisms for the modification of numerous processes, including energy metabolism, mitochondrial function, oxidative damage, and neuroendocrine homeostasis (2), giving rise to diverse hypotheses on how CR may extend lifespan by a variety of mechanisms (2, 3, 6, 7). Aging also results in profound changes in the immune system (immune senescence) (8, 9), resulting in a dysregulation of both the innate and adaptive arms of the immune response, which normally ensure optimal protection against pathogens (10). Consequently, infectious diseases are among the leading causes of morbidity and mortality in the elderly (11, 12). The fact that CR can partially retard or reverse some of the changes associated with immune senescence in rodents provides the basis for the hypothesis that improvement of the immune function by CR contributes to extended lifespan by affording higher resistance to pathogens (8, 9).
Within the adaptive immune system, T cells are probably the component most affected by immune senescence. Diminished T cell function (13); decreased production of new, naïve T cells by the thymus (14); increased homeostatic turnover of naïve T cells (15); and lifelong consumption of naïve cells due to pathogenic assaults all contribute to a decline in T cell immunity. The loss of naïve lymphocytes leads to a decrease in T cell receptor (TCR) repertoire diversity, which is further compounded by the appearance of T cell clonal expansions (16–19) that additionally constrict normal lymphocyte repertoire and can potentially interfere with protective immunity (20). Rodent studies suggest that CR can reduce the rate of immune senescence, preserve naïve T cells into late age (21), and reduce the production of proinflammatory cytokines such as IL-6 and TNF-α (22).
Given the robust results reported in short-lived species, long-term studies have been initiated in the Old World nonhuman primate rhesus macaque (Macaca mulatta; RM) model to test the impact of CR on mortality, morbidity, and age-related changes in the function of various cells, tissues, and organ systems (23, 24). Shortly after the initiation of CR in RMs in the National Institute on Aging (NIA) study (summarized in ref. 25), it was reported that CR-treated RMs displayed physiological changes similar to those observed in CR rodents, specifically lower body weight, fat mass, temperature, and circulating plasma insulin levels. CR was also found to maintain dehydroepiandrosterone sulfate (DHEAS) and melatonin, two of the biomarkers of human aging, at youthful levels in RMs (25, 26). Moreover, preliminary studies in nonhuman primates suggest that mortality due to diabetes, cardiovascular disease, and cancer might be lower in CR animals than in controls (23). Similar results were reported in a cohort of CR monkeys at the University of Wisconsin (24). By contrast, studies measuring immunological parameters in unseparated peripheral blood mononuclear cells (PBMC) of adult and aged monkeys on CR for 2–4 years (27–30) failed to find any beneficial outcomes of CR or reported opposite results compared with CR-treated rodents. Therefore, the exact effects of long-term CR on defined cell subsets of the immune system in aged RMs remain poorly understood. To test the impact of CR on T cell senescence in RMs, we investigated T cell subset distribution, TCR repertoire diversity, thymic production of new T cells, and peripheral T cell function in 19- to 23-year-old male and female monkeys subjected to CR or control (CON) treatments for 13–18 years. Given that the estimated median lifespan of RMs is 25 years (40 years maximal), monkeys in this study represent a human age equivalent of ≈60–70 years. Our results show remarkable conservation of naïve T cell phenotype and function by CR in long-lived primates.
Results
Overall Study Design and Animal Cohorts.
Experimental cohorts consisted of aged CON and CR RMs of both sexes that were 19–23 years of age during the course of sampling for this study. The study animals are described in detail in Table 1 and in Materials and Methods, and their diet and husbandry are described in ref. 31. We obtained samples for analysis for all of the available animals in the cohort at four time points over 42 months. [For a note on attrition, see supporting information (SI) Discussion.] The time points and numbers of animals sampled were as follows: males: 11/2002 (7 CR, 19 CON), 05/2003 (7 CR, 18 CON), 07/2004 (7 CR, 18 CON), and 05/2005 (7CR, 18CON); females: 02/2003 (6 CR, 10 CON), 07/2004 (5 CR, 9 CON), 03/2005 (4 CR, 8 CON), and 09/2005 (4 CR, 8 CON). The main reason for female attrition was endometriosis, a condition that is difficult to diagnose and problematic to treat in female RMs and that peaks between 20 and 25 years of age. Samples were counted, frozen by controlled cryopreservation, and subsequently thawed for analysis, allowing simultaneous analysis of samples collected at disparate time points. Time on the CR diet was different for the male and female cohorts due to the different lengths of time they were enrolled in the study (14–17 years for males and 10–13 years for females; spread due to the longitudinal observation over the 42-month period of the study), did not statistically influence any of the parameters analyzed (data not shown), and was not considered separately. However, female and male groups are shown separately to preserve power for statistical considerations, because sex-related differences in immunological parameters introduced additional variability. Regardless of these baseline sex-related differences in CON animals, CR exhibited strong sex-independent effects on the immune system of aging animals throughout these studies.
Table 1.
Animal groups and blood cell counts
| Group | No. of animals | Age, yr | Cell type |
||
|---|---|---|---|---|---|
| WBC | LYM | NE | |||
| Males | |||||
| CON | 18 | 20–23 | 3.93 ± 0.31 | 1.97 ± 0.15 | 1.48 ± 0.26 |
| CR | 7 | 20–23 | 4.1 ± 0.38 | 2.13 ± 0.19 | 1.38 ± 0.34 |
| Females | |||||
| CON | 10 | 20–21 | 4.7 ± 0.44 | 1.73 ± 0.20 | 2.46 ± 0.36 |
| CR | 7 | 19–21 | 4.24 ± 0.41 | 2.14 ± 0.19 | 1.73 ± 0.34 |
The number of white blood cells (WBC), lymphocytes (LYM), and neutrophils (NE) was determined by using the Beckman Coulter counter calibrated for RM cells. Animals were sampled over 42 months, and data shown represent aggregate mean values (± SEM) of all six time points. No significant sex-related differences in blood cell counts were observed between the CON and CR groups.
For our analysis, a group average (CON males, CR males, CON females, and CR females) was estimated at each of the time points by using the mixed-effects model, as described in Materials and Methods, and was compared between time points. We observed no significant differences between the time points for the same group (data not shown), and the differences between the CON and CR groups, as described below, were apparent at all time points examined, leading us to conclude that, by the earliest time point of sampling (10 years on CR in females) the effects of long-term CR were fully established. This allowed us to include all time points into our analysis, so as to average group mean values between time points and then perform statistical analysis on those values by using mixed-effect models, as described in Materials and Methods.
Impact of CR on Blood Counts and T Cell Phenotype.
In rodents, CR was reported to cause a state of lymphopenia, and initial studies conducted 2 years after the onset of CR in adult RMs and adult humans suggested a slight decrease in circulating WBC (28, 32). By contrast, complete blood cell count analysis of RMs in this study (at six time points over 3 years) revealed that total WBC, lymphocyte, and neutrophil counts in CR animals were similar to those observed in CON animals, with no significant sex-related differences (Table 1). Therefore, contrary to the results in rodents, long-term CR did not induce a lifelong leuko- or lymphopenic state in RMs. These results, however, do not rule out the possibility that during the CR induction period and thereafter, lymphopenia could occur in RMs adapting to a new nutritional status.
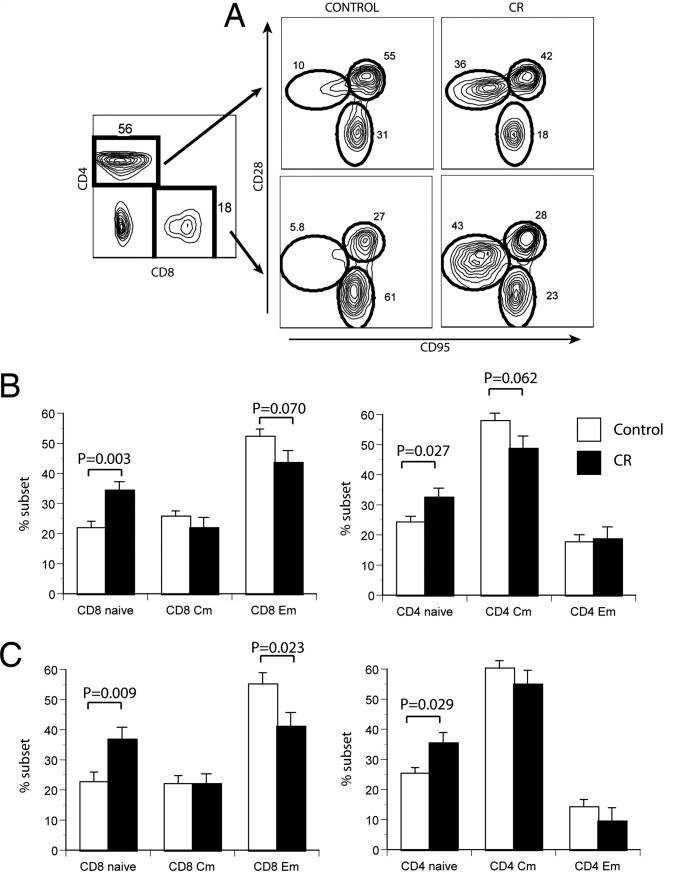
We next examined the phenotype distribution of T cell subsets in PBMC from CON and CR animals. We classified CD28intCD95lo cells as naïve (N), CD28hiCD95hi cells as central (resting) memory (CM), and CD28lo/negCD95hi cells as effector/effector memory (EM) for both CD4 and CD8 subsets, as described previously (33) (Fig. 1A). The ratio of naïve to memory T cells decreases reproducibly with age in many mammals, including RMs (33, 34, and our unpublished results), so that naïve cells decline from >40% in adult animals to <20% of total blood T cells in most aged RMs (Fig. 1). CR lessened this decline and maintained a higher percentage of both CD8 and CD4 naïve T cells independent of sex (Fig. 1). In the CD8 subset, this increase occurred mostly at the expense of the EM T cells, whereas in the CD4 subset naïve cells appeared to increase perhaps more at the expense of CM cells. Of importance, the above changes were absolute; total lymphocyte numbers did not change significantly with CR (Table 1) or age (data not shown) in the blood of RMs we have studied.
Fig. 1.
Phenotypic changes in aged RM T cells as a consequence of CR. (A) Example of four-color flow cytofluorometry analysis of PBMC from a representative pair of age-matched CON and CR animals. PBMC were stained with antibodies against CD8β, CD4, CD28, and CD95; were gated on CD8β or CD4 populations (Left); and were analyzed for the expression of CD28 and CD95 (Right). Circles denote N, CM, and EM subsets (encompassing >94% of all cells), as described in the text; numbers denote the percentage of cells in each population. (B and C) Cumulative percentages of T cell subsets were analyzed in all CON (open histograms) and CR (filled histograms) RM males (B) and females (C) over the four different analysis time points over a span of 42 months, as described in the text. Mean values are shown for each group at four time points, calculated from mean values at each time point ± SEM. Statistical analysis was performed using the mixed-effects model approach, and significance is indicated by two-sided P values above the histograms.
Analysis of Recent T Cell Emigrants and of T Cell Repertoire Under CR Treatment.
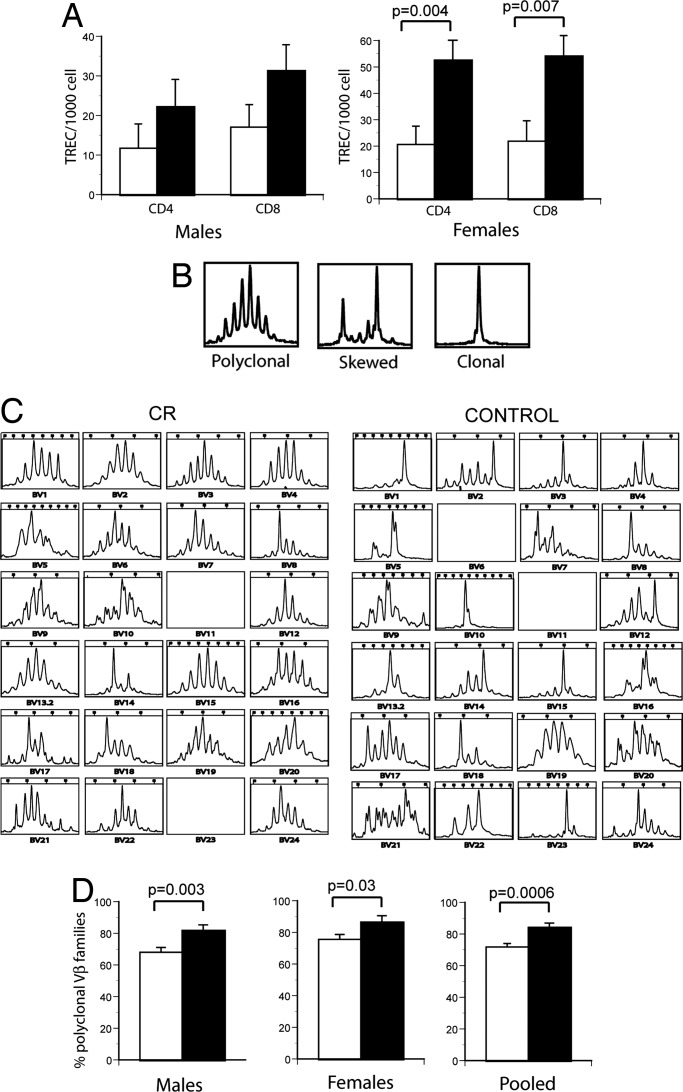
To independently confirm that the numbers of naïve T cells were higher in CR-treated animals, we measured in the PBMC of CON and CR animals the content of TCR excision circles (TREC), as a marker of recently produced T cells (recent thymic emigrants) or T cells that did not divide after being produced by the thymus, as described previously (35). Both CD4 and CD8 T cells from male and female CR-treated RMs exhibited higher TREC content per 103 cells compared with CON counterparts (borderline significance in males; significant in females; Fig. 2A).
Fig. 2.
TCR repertoire analysis and TREC analysis of CON and CR T cells. (A) Estimation of recent thymic emigrants, using the TREC assay, was done as described in ref. 32. Results are shown as number of TREC per 103 T cells. (B) Representative examples of polyclonal (Left), skewed (Center), and clonal (Right) patterns of TCR CDR3 length polymorphism. (C) Example of CDR3 length analysis for all 24 TCRVβ families for representative age-matched CON and CR animals, performed as described in Materials and Methods. (D) Cumulative analysis (all animals) of CDR3 length polymorphisms in CON (open histograms) and CR (filled histograms) animals. For both A and D, statistical analysis was performed using the mixed-effects model.
Increased representation of naïve T cells in peripheral blood would be expected to increase TCR diversity in that population. Consequently, TCR diversity was examined by measuring CDR3 length polymorphism (36) within peripheral blood T cells from CR and CON RMs. Fig. 2B summarizes examples of polyclonal, diverse CDR3 length profiles (typical Gaussian distribution, Fig. 2B Left), as well as distorted profiles (skewed, Fig. 2B Center; and oligo/monoclonal, Fig. 2B Right), which are characteristic of expanded T cell clones. Such expanded clones frequently occur in aged individuals (as reviewed in ref. 37), in which homeostatic compensation and chronic or lifelong exposure to antigens likely synergize to reduce T cell diversity (15, 38). Indeed, in aged CON RMs, TCRVβ CDR3 length polymorphism analysis revealed that, of 22 successfully amplified Vβ families, only 4 had polyclonal appearance (Vβ4, 9, 19, and 20), whereas 5 (Vβ1, 5, 10, 15, and 23) exhibited oligo- or monoclonal profiles. By contrast, an age-matched CR animal exhibited Gaussian profiles for at least 14 Vβ families (Vβ1–6, 9, 10, 13, 15, 16, 19, 20, and 24), with perhaps only one profile (Vβ14) being oligoclonal (Fig. 2C). Results of cumulative analysis revealed significantly higher diversity in both male and female CR animals (Fig. 2D), demonstrating that the increase in naïve phenotype T cells with CR, seen in Fig. 1, led to increased TCR diversity in peripheral blood.
Naïve-phenotype cells, defined as in Fig. 1, did not produce any cytokines upon TCR stimulation in the short-term intracellular cytokine staining assay (see Fig. 4A and ref. 33) and expressed other phenotypic characteristics of naïve cells, being CCR7+ and CD62Lhi (data not shown). Overall, we conclude that CR countered the effects of aging on T cell subsets by increasing or maintaining the youthful levels of bona fide naïve T cells and that this could in part be due to increased thymic production. However, because naïve T cells possess the capacity for self-renewal without overt changes in membrane phenotype (39), CR may also contribute to better maintenance by slowing the turnover of these cells.
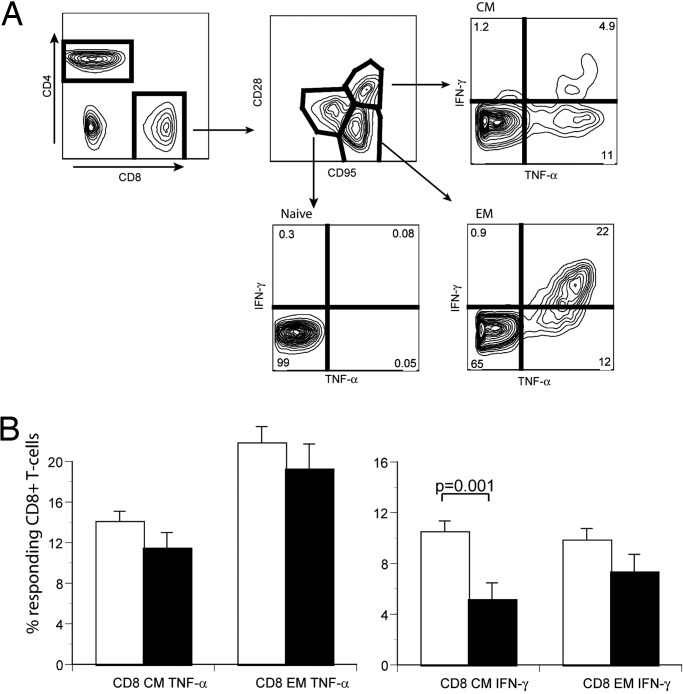
Fig. 4.
Production of proinflammatory cytokines by T cell subsets of CON and CR animals. (A) Illustration of flow cytofluorometry assay to measure ex vivo cytokine production by T cells. Blood T cells were stimulated for 6 h with an agonistic anti-TCR antibody, as described in Materials and Methods, and stained to reveal expression of CD4, CD8, CD95, CD28, and intracellular TNF-α. Selective gating is depicted by arrows and shows production of TNF-α by the N, CM, and EM subsets. (B) Cumulative analysis of TNF-α (Left) and IFN-γ (Right) in CD8 CM and EM T cell subsets of CON (open histograms) and CR (filled histograms) male monkeys. Statistical analysis was performed on all male animals as described in Fig. 1; significance is indicated by two-sided P values.
Impact of CR on T Cell Function in Aged Monkeys.
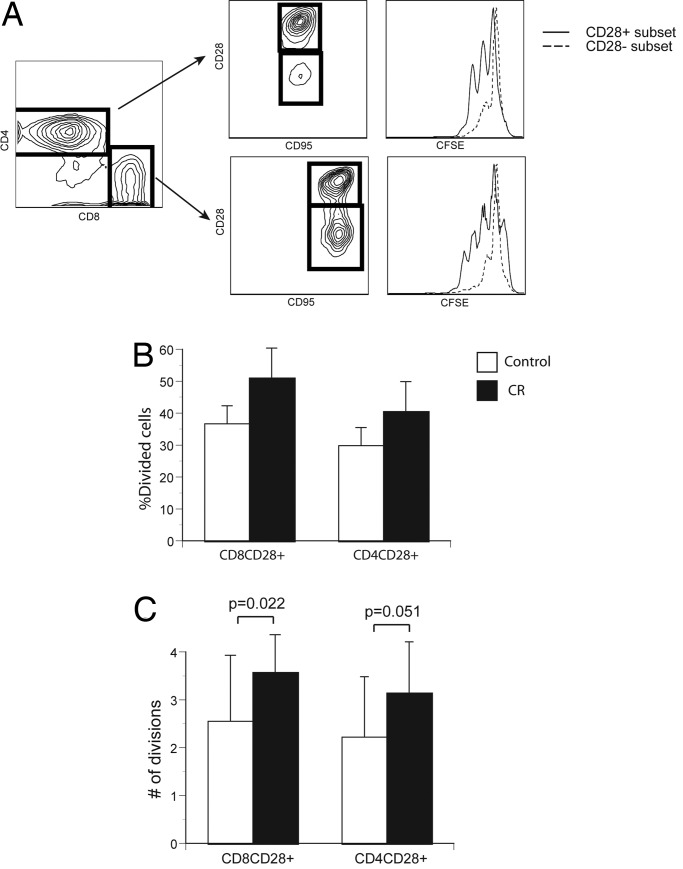
Immune senescence is accompanied by a decrease in the proliferative potential of T cells, likely due to both an accumulation of terminally senescent cells and an age-related reduction in the proliferative ability of naïve cells (38, 40). Although CR was reported to ameliorate these proliferative defects in response to mitogens in aged rodents (41), initial reports in aged CR-treated male RMs suggested unchanged or lower responsiveness to different mitogens (27, 28). We used fluorescein label (CFSE) dilution to enumerate precisely the number of divisions in response to TCR stimulation in male CR- or CON-treated T cells in a 96-h stimulation assay. We confirmed that CD28+ cells respond to stimulation by vigorous proliferation, whereas CD28− cells were far less active (Fig. 3A), as expected from previous data on human CD28− T cells (42). CR increased the percentage of male CD28+CD4+ and CD28+CD8+ cells that divided at least once at every time point analyzed (Fig. 3B), but this trend did not reach statistical significance. However, when the average number of divisions completed was analyzed (Fig. 3C), results were significant for CD28+CD8+ cells and borderline for CD28+CD4+ cells. This could be due to a higher content of naïve proliferating T cells, better proliferation of CM T cells, removal of senescent cells by CR, or a combination thereof. Regardless, these results demonstrate that CR leads to improved proliferation of aged male RM T cells. Female T cells analyzed at the first time point of this study followed the trend of the male cells. Subsequently, nearly half of the female CR group was lost to attrition, severely reducing the power of the study. Consequently, additional studies are needed to extend these conclusions to both sexes.
Fig. 3.
Proliferation of CON- and CR-treated T cells in response to an agonistic antibody. (A) Illustration of flow cytofluorometry profiles of stimulated fluorescein (CFSE)-labeled CD4 and CD8 cells (stained and gated as described in Fig. 1) from a CON animal. Left shows CD4/CD8 profiles; Center shows CD28/95 profiles of gated cells from left; and Right depicts the successive fluorescein dilution in each proliferating subset at 96 h after stimulation. (B) Percentage of divided CD28+ T cells in CON (open histograms) and CR (filled histograms) males. Results are shown as mean ± SEM from a representative experiment out of three with comparable results. (C) Average number of divisions in the CD8+CD28+ and CD4+ CD28+ T cell subsets of CON (open histograms) or CR (filled histograms) male monkeys. All male animals were analyzed, and statistical analysis was as described in Fig. 1.
Finally, we addressed the influence of CR on the immediate cytokine secretion capacity of T cell subsets. Immune senescence in mammals, including primates, is accompanied by an increase in the levels of proinflammatory cytokines, including the interferons, IL-6, and TNF-α (34, 43, 44). Increased and poorly controlled inflammatory reactions have been associated with numerous organ-specific pathologies, including coronary vascular disease, dementias, neurodegenerative disorders, type II diabetes, and arthritis (45). We performed brief stimulation of T cells from CON and CR animals by means of the TCR/CD3 complex and measured production of intracellular IFN-γ and TNF-α. After such stimulation, only CM and EM cells produced cytokines (Fig. 4A). However, fewer T cells from CR-treated animals produced both cytokines compared with their CON counterparts (Fig. 4B and SI Fig. 5). The effect showed a consistent trend at all time points examined (four time points, represented in aggregate in SI Fig. 5), but was not significant in females, possibly because the age-related increase in T cell cytokine production was less pronounced in females. Results were of borderline significance in male CD4 cells for TNF-α and were significant in male CD4 cells for IFN-γ (SI Fig. 5). In light of phenotype data (Fig. 1), this effect could be explained by a higher proportion of naïve cells in CR-treated RMs. Because these cells do not produce cytokines under short-term stimulation (Fig. 4A), they would simply dilute memory cells that produce cytokines. However, CR had a direct effect on memory cells as well. Upon subdivision of male CD8 cells into memory subsets, it became apparent that CR mediated a highly significant reduction in CM cells producing IFN-γ, whereas the reduction in EM cells was consistent but did not reach statistical significance (Fig. 4B). A similar trend was observed for TNF-α (Fig. 4B) and was also seen at the first time point with female animals, before power was lost to attrition (data not shown). Overall, these results strongly suggest that CR down-regulates baseline cytokine production by CM T cells.
Discussion
In this study, we provide evidence for several beneficial effects of long-term CR on the immune system of aged nonhuman primates. T cell population balance and T cell subset function were both modulated toward values usually seen in younger adult animals. Thus, long-term CR initiated early in adulthood resulted in higher percentages of naïve T cells and lower percentages of memory T cells in the circulating PBMC of aged nonhuman primates. These cells in CR animals were not only phenotypically, but also functionally, naïve. This is notable because a higher prevalence of naïve lymphocytes bearing a diverse TCR repertoire should preserve immune reactivity to new antigens encountered late in life. In that regard, it has been demonstrated that TCR diversity is important in immune defense (7) and that its decline with aging can lead to impaired resistance to infection and even to death (20). Inasmuch as susceptibility to infectious diseases is among the top five leading causes of mortality and morbidity in elderly patients, a more efficient immune response in aged individuals is likely to increase lifespan. In that regard, Miller and colleagues (46, 47) demonstrated that in genetically heterogeneous mice, the percentage of blood CD4 memory T cells, followed by the percentage of blood CD8 memory cells, measured at 18 months of life, was the most reliable inverse predictor of longevity. Further studies suggested that the pace and/or extent of memory T cell accumulation might also be a reliable predictor of longevity in rodents (47). Similar studies in humans have shown a strong association between chronic pathogens that induce progressive accumulation of memory T cells (particularly CMV) and shorter lifespans in advanced aging (38). It should be noted that all monkeys in this study were CMV+. The ability of CR to reduce the numbers of memory cells and increase the numbers of naïve T cells in nonhuman primates is consistent with the idea that, even in the face of chronic infection, CR is capable of ameliorating immunological aging and inhibiting immune exhaustion. However, an alternative explanation that CR may reduce reactivation or replication of persistent viruses cannot be ruled out at present. Unfortunately, because RMs continuously shed CMV in mucosal fluids (48), it is very difficult to measure viral reactivation in this model. The final test of CR value to immunity, of course, will lie in definitive experiments to demonstrate whether CR animals are indeed more resistant to pathogenic challenge.
Long-term CR also reduced immediate, ex vivo cytokine secretion by stimulated memory T cells. This contrasts with the recent finding that 2 years of CR actually increased production of IFN-γ by PBMC (30) in another group of aged male RMs. Differences in stimulation type and length, cell population, duration of CR, or monkey cohort could, individually or together, account for the discrepancy between the two studies. Regardless, our findings strongly suggest that lifelong CR may have anti-inflammatory effects in primate T cells. Given the association of the proinflammatory state with numerous chronic diseases that are prevalent in old age, this effect would also be likely to contribute to improved health and longevity in CR animals. Clearly, a balance needs to exist between stimulating adequate inflammatory and immune responses in the face of immediate pathogen assault and dampening and controlling the chronic responses, with potential to inflict immunopathology. In that regard, our preliminary results on other RM cohorts kept on CR for 8–9 years indicate that, in vivo, CR enables the maintenance of robust primary immune responses. Meanwhile, in the same cohort, the reactivity to a chronic pathogen, CMV, was not appreciably different between CR and CON animals. If confirmed, this would suggest that reduction of baseline T cell cytokine secretion does not adversely impact the ability to react vigorously to new antigenic challenge, an issue critically important to immune defense and longevity.
The key remaining challenge is to explain, at the molecular and cellular levels, the mechanisms of CR action on diverse organs and tissues. Our studies show that at least part of the CR effect lies in the increased production and/or slower turnover of naïve T cells, based on increased TREC levels. Because inflammatory cytokines tend to reduce thymic output by inducing apoptosis in immature thymocytes (14), CR could increase thymic output by reducing inflammatory cytokine levels. However, this effect could also occur at other steps of hematopoietic and/or early T cell lineage development, and it may also involve improved function of thymic stromal cells. As samples from the animals studied here become available at necropsy, it may be possible to address some of these questions. Moreover, molecular analysis of gene and protein expression patterns from primary and secondary lymphoid tissues, as well as from defined, phenotypically and functionally homogenous lymphocyte subsets, should provide additional clues regarding the impact of CR on the immune system.
Materials and Methods
Animals.
Monkeys in this study were part of an ongoing study of aging and CR in RMs at the NIA and were housed continuously at the Primate Unit of the National Institutes of Health (NIH) Veterinary Research Program, Poolesville, MD. Diet composition and animal husbandry procedures were as described in ref. 31. Experimental cohorts (Table 1) included 13 CR (7 male and 6 female) and 28 CON (18 male and 10 female) rhesus monkeys 19–23 years of age. Over the 42 months of examination, attrition reduced these numbers by one CON male, two CON females, and two CR females (see SI Discussion). We obtained complete cohort samples at four time points over the 42-month period and, unless otherwise indicated, have used all four time points to perform statistical analysis and draw our conclusions (see below).
Sample Collection, Flow Cytometry, and Functional Assays.
After an overnight fast, peripheral blood was collected within 30 min of anesthesia into heparinized tubes, as described in refs. 33 and 34. Whole blood was analyzed with a Beckman Coulter (Hialeah, FL) complete blood count machine. PBMCs were isolated from whole blood after centrifugation over Ficoll–Histopaque (Sigma, St Louis, MO). Cells were either analyzed immediately or were frozen for subsequent analysis by using a controlled cyropreservation apparatus procedure (CryoMed Freezer no. 7454; ThermoForma, Marietta, OH), with no significant differences seen between the analyzed properties of fresh and frozen/thawed cells. All antibodies were purchased from PharMingen (San Diego, CA), Ebioscience (San Diego, CA), or Caltag (Burlingame, CA) and were used in accordance with manufacturer's recommendations. Samples were collected using FACSCalibur or FACSLSRII (Becton Dickinson, San Jose, CA), and data were analyzed using CellQuest (BD Biosciences, Mountain View, CA) or FlowJo (Treestar, Ashland, OR), with a minimum of 105 events collected per sample. Proliferation assay and intracellular cytokine staining were performed exactly as described in ref. 34.
CDR3 Length and TREC Analysis.
RNA was isolated from 3 × 106 to 5 × 106 PBMC by using RNAisolator (Sigma). cDNA reverse transcription, TCRVβ-specific PCRs, and runoff labeling with fluorescent primers were performed as described previously (49), using the primers described in ref. 50.
Quantification of TREC in DNA of PBMC was done by quantitative–competitive PCR exactly as described in ref. 35. DNA was extracted from the cells, and 1 μg was added to each PCR, which also contained 102, 103, or 104 molecules of an internal competitor standard. Primers and conditions were as described in ref. 35. The reactions were run for 35 cycles and incorporated 32P-labeled dCTP. PCR products were separated on nondenaturing 6% polyacrylamide gels. Bands were imaged and analyzed with a Cyclone storage phosphor system and Optiquant software (Packard, Meriden, CT).
Statistical Analysis.
Because the parameters on all animals were measured at multiple time points over time (four times over 42 months, with no measurement closer than 6–7 months to another measurement), mixed-effects models were used to analyze whether the CR and CON groups had similar temporal changes in WBC, percentage of naïve cells, percentage of cells divided, and cytokine secretion. The covariance structure was assumed to have a compound symmetry based on the Bayesian information criterion (51). TREC data were analyzed by one-way ANOVA. The data on the number of divisions were assumed to have Poisson distribution and were analyzed by using the generalized estimating equations (GEE) method (52). All statistical analyses were performed using Statistical Analysis System software (SAS version 9.0; SAS Institute, Cary, NC), and P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank April Hobbs and Ed Tilmont (NIA Intramural Research Program, Poolesville, MD) for their work and dedication, Drs. Doug Powell and Rick Herbert (NIH Veterinary Research Program, Poolesville, MD) for the veterinary care of the monkeys in this study and for collection of the specimens, Dr. John Fanton [Oregon National Primate Research Center (ONPRC)] for surgical assistance, and Dr. Steven Kohama (ONPRC) for helpful discussion. This work was supported in part by U.S. Public Health Service Awards AG21384 (from the NIA to J.N.-Ž.), 5T32 AI007472-10 [from the National Institute of Allergy and Infectious Diseases (NIAID) to I.M.], and RR0163 (from the National Institute for Research Resources to ONPRC), as well as by the NIA and NIAID intramural programs.
Abbreviations
- CM
central memory T cells
- CON
control
- CR
caloric restriction
- EM
effector/effector memory T cells
- N
naïve T cells
- PBMC
peripheral blood mononuclear cells
- RM
rhesus macaque (Macaca mulatta)
- TCR
T cell receptor
- TREC
TCR excision circles.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0606661103/DC1.
References
- 1.McCay CM, Crowell MF, Maynard LA. Nutrition. 1989;5:155–171. discussion 172. [PubMed] [Google Scholar]
- 2.Masoro EJ. Mech Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Heilbronn LK, Ravussin E. Am J Clin Nutr. 2003;78:361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- 4.Hursting SD, Lavigne JA, Berrigan D, Donehower LA, Davis BJ, Phang JM, Barrett JC, Perkins SN. J Nutr. 2004;134:2482S–2486S. doi: 10.1093/jn/134.9.2482S. [DOI] [PubMed] [Google Scholar]
- 5.Guarente L, Picard F. Cell. 2005;120:473–482. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 6.Sinclair DA. Mech Ageing Dev. 2005;126:987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Messaoudi I, Guevara Patino JA, Dyall R, LeMaoult J, Nikolich-Žugich J. Science. 2002;298:1797–1801. doi: 10.1126/science.1076064. [DOI] [PubMed] [Google Scholar]
- 8.Walford RL. J Am Geriatr Soc. 1982;30:617–625. doi: 10.1111/j.1532-5415.1982.tb05057.x. [DOI] [PubMed] [Google Scholar]
- 9.Miller RA. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 10.Nikolich-Žugich J. J Exp Med. 2005;201:837–840. doi: 10.1084/jem.20050341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gardiner I. Rev Infect Dis. 1980;3:2801–2814. [Google Scholar]
- 12.Wick G, Jansen-Durr P, Berger P, Blasko I, Grubeck-Loebenstein B. Vaccine. 2000;18:1567–1583. doi: 10.1016/s0264-410x(99)00489-2. [DOI] [PubMed] [Google Scholar]
- 13.Miller RA, Garcia G, Kirk CJ, Witkowski JM. Immunol Rev. 1997;160:79–90. doi: 10.1111/j.1600-065x.1997.tb01029.x. [DOI] [PubMed] [Google Scholar]
- 14.Linton PJ, Dorshkind K. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 15.Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ. J Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 16.Callahan JE, Kappler JW, Marrack P. J Immunol. 1993;151:6657–6669. [PubMed] [Google Scholar]
- 17.Hingorani RC, Choi IH, Akolkar P, Gulwani-Akolkar B, Pergolizzi R, Silver J, Gregersen PK. J Immunol. 1993;151:5762–5769. [PubMed] [Google Scholar]
- 18.Posnett DN, Sinha S, Kabak S, Russo C. J Exp Med. 1994;179:609–617. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeMaoult J, Szabo P, Weksler ME. Immunol Rev. 1997;160:115–126. doi: 10.1111/j.1600-065x.1997.tb01032.x. [DOI] [PubMed] [Google Scholar]
- 20.Messaoudi I, LeMaoult J, Guevara-Patino JA, Metzner BM, Nikolich-Žugich J. J Exp Med. 2004;200:1347–1358. doi: 10.1084/jem.20040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Astle CM, Harrison DE. J Gerontol A Biol Sci Med Sci. 1998;53:B330–B337. doi: 10.1093/gerona/53a.5.b330. [DOI] [PubMed] [Google Scholar]
- 22.Jolly CA. J Nutr. 2004;134:1853–1856. doi: 10.1093/jn/134.8.1853. [DOI] [PubMed] [Google Scholar]
- 23.Mattison J, Lane M, Roth G, Ingram D. Exp Gerontol. 2003;38:35–46. doi: 10.1016/s0531-5565(02)00146-8. [DOI] [PubMed] [Google Scholar]
- 24.Ramsey JJ, Colman RJ, Binkley NC, Christensen JD, Gresl TA, Kemnitz JW, Weindruch R. Exp Gerontol. 2000;35:1131–1149. doi: 10.1016/s0531-5565(00)00166-2. [DOI] [PubMed] [Google Scholar]
- 25.Lane MA, Black A, Handy A, Tilmont EM, Ingram DK, Roth GS. Ann NY Acad Sci. 2001;928:287–295. doi: 10.1111/j.1749-6632.2001.tb05658.x. [DOI] [PubMed] [Google Scholar]
- 26.Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, Tobin JD, Muller D, Metter EJ. Science. 2002;297:811. doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- 27.Roecker EB, Kemnitz JW, Ershler WB, Weindruch R. J Gerontol A Biol Sci Med Sci. 1996;51:B276–B279. doi: 10.1093/gerona/51a.4.b276. [DOI] [PubMed] [Google Scholar]
- 28.Weindruch R, Lane M, Ingram D, Ershler WB, Roth G. Aging Clin Exp Res. 1997;9:304–308. doi: 10.1007/BF03341833. [DOI] [PubMed] [Google Scholar]
- 29.Grossmann A, Rabinovitch PS, Lane MA, Jinneman JC, Ingram DK, Wolf NS, Cutler RG, Roth GS. J Cell Physiol. 1995;162:298–303. doi: 10.1002/jcp.1041620216. [DOI] [PubMed] [Google Scholar]
- 30.Mascarucci P, Taub D, Saccani S, Paloma MA, Dawson H, Roth GS, Lane MA, Ingram DK. J Interferon Cytokine Res. 2002;22:565–571. doi: 10.1089/10799900252982043. [DOI] [PubMed] [Google Scholar]
- 31.Ingram DK, Cutler RG, Weindruch R, Renquist DM, Knapka JJ, April M, Belcher CT, Clark MA, Hatcherson CD, Marriott BM, Roth GS. J Gerontol. 1990;45:B148–B163. doi: 10.1093/geronj/45.5.b148. [DOI] [PubMed] [Google Scholar]
- 32.Walford RL, Mock D, Verdery R, MacCallum T. J Gerontol A Biol Sci Med Sci. 2002;57:B211–B224. doi: 10.1093/gerona/57.6.b211. [DOI] [PubMed] [Google Scholar]
- 33.Pitcher C, Hagen S, Walker J, Lum R, Mitchell B, Maino V, Axthelm M, Picker LJ. J Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 34.Janković V, Messaoudi I, Nikolich-Žugich J. Blood. 2003;102:3244–3251. doi: 10.1182/blood-2003-03-0927. [DOI] [PubMed] [Google Scholar]
- 35.Sodora D, Douek DC, Silvestri G, Montgomery L, Rosenzweig M, Igarashi T, Bernacky B, Johnson RP, Feinberg MB, Martin MA, Koup RA. Eur J Immunol. 2000;30:1145–1153. doi: 10.1002/(SICI)1521-4141(200004)30:4<1145::AID-IMMU1145>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 36.Pannetier C, Even J, Kourilsky P. Immunol Today. 1995;16:176–181. doi: 10.1016/0167-5699(95)80117-0. [DOI] [PubMed] [Google Scholar]
- 37.Clambey ET, van Dyk LF, Kappler JW, Marrack P. Immunol Rev. 2005;205:170–189. doi: 10.1111/j.0105-2896.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 38.Pawelec G, Akbar A, Caruso C, Effros R, Grubeck-Loebenstein B, Wikby A. Trends Immunol. 2004;25:406–410. doi: 10.1016/j.it.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Tough DF, Sprent J. J Exp Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Effros RB. Exp Gerontol. 2004;39:885–890. doi: 10.1016/j.exger.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 41.Walford RL, Liu RK, Gerbase-Delima M, Mathies M, Smith GS. Mech Ageing Dev. 1973;2:447–454. doi: 10.1016/0047-6374(73)90035-3. [DOI] [PubMed] [Google Scholar]
- 42.Effros RB. Dev Comp Immunol. 1997;21:471–478. doi: 10.1016/s0145-305x(97)00027-x. [DOI] [PubMed] [Google Scholar]
- 43.Ershler WB, Sun WH, Binkley N. Drugs Aging. 1994;5:358–365. doi: 10.2165/00002512-199405050-00005. [DOI] [PubMed] [Google Scholar]
- 44.Zanni F, Vescovini R, Biasini C, Fagnoni F, Zanlari L, Telera A, Di Pede P, Passeri G, Pedrazzoni M, Passeri M, et al. Exp Gerontol. 2003;38:981–987. doi: 10.1016/s0531-5565(03)00160-8. [DOI] [PubMed] [Google Scholar]
- 45.De Martinis M, Franceschi C, Monti D, Ginaldi L. FEBS Lett. 2005;579:2035–2039. doi: 10.1016/j.febslet.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 46.Miller RA. Mech Ageing Dev. 1997;96:181–196. doi: 10.1016/s0047-6374(97)01893-9. [DOI] [PubMed] [Google Scholar]
- 47.Harper JM, Galecki AT, Burke DT, Miller RA. Mech Ageing Dev. 2004;125:381–390. doi: 10.1016/j.mad.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Huff JL, Eberle R, Capitanio J, Zhou SS, Barry PA. J Gen Virol. 2003;84:83–92. doi: 10.1099/vir.0.18808-0. [DOI] [PubMed] [Google Scholar]
- 49.Pannetier C, Cochet M, Darche S, Casrouge A, Zoller M, Kourilsky P. Proc Natl Acad Sci USA. 1993;90:4319–4323. doi: 10.1073/pnas.90.9.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Z, Li Y, Zeng X, Kuroda M, Schmitz J, Shen Y, Lai X, Shen L, Letvin N. J Immunol. 2001;166:4525–4533. doi: 10.4049/jimmunol.166.7.4525. [DOI] [PubMed] [Google Scholar]
- 51.Box GEP, Jenkins GM, Reinsel GC. Time Series Analysis: Forecasting and Control. Englewood Cliffs, NJ: Prentice–Hall; 1994. [Google Scholar]
- 52.Zeger SL, Liang KY. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.