Abstract
Amyotrophic lateral sclerosis (ALS) is a fatal, progressive paralysis arising from the premature death of motor neurons. An inherited form is caused by a dominant mutation in the ubiquitously expressed superoxide dismutase (SOD1). SOD1 mutant expression within motor neurons is a determinant of onset and early disease, and mutant accumulation within microglia accelerates disease progression. Muscle also is a likely primary source for toxicity, because retraction of motor axons from synaptic connections to muscle is among the earliest presymptomatic events. To test involvement of muscle in ALS, viral delivery of transcription-mediated siRNA is shown to suppress mutant SOD1 accumulation within muscle alone but to be insufficient to maintain grip strength, whereas delivery to both motor neurons and muscle is sufficient. Use of a deletable mutant gene to diminish mutant SOD1 from muscle did not affect onset or survival. Finally, follistatin expression encoded by adeno-associated virus chronically inhibited myostatin and produced sustained increases in muscle mass, myofiber number, and fiber diameter, but these increases did not affect survival. Thus, SOD1-mutant-mediated damage within muscles is not a significant contributor to non-cell-autonomous pathogenesis in ALS, and enhancing muscle mass and strength provides no benefit in slowing disease onset or progression.
Keywords: siRNA, adeno-associated virus, motor neuron, G93A SOD1, myostatin
Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig's disease in the United States, is an adult-onset disease that causes degeneration of motor neurons. The loss of motor neurons causes various degrees of weakness and atrophy of limb muscles with concomitant dysphagia, dysarthria, and spasticity. Death usually occurs in 2–5 years and is related to respiratory weakness. Ten percent of ALS is familial, and one-fifth of these familial cases are caused by mutations in superoxide dismutase 1 (SOD1). Transgenic mice and rats expressing a mutant human SOD1 transgene develop an ALS phenotype, whereas absence of SOD1 does not cause motor neuron disease, providing evidence for an acquired toxicity of mutant SOD1 (1).
Despite extensive research using mice and rats expressing mutant SOD1 that recapitulate the familial form of ALS, the exact nature of the underlying toxicity of mutant SOD1 remains unknown (2). However, it is becoming increasingly clear that cells other than the motor neurons themselves contribute to the demise of the motor neurons. By using animals chimeric for mutant SOD1 expression, mutant SOD1 motor neurons can be rescued by normal non-neuronal cells, and, conversely, normal motor neurons can acquire toxicity from neighboring mutant SOD1 cells (3). Furthermore, reducing mutant SOD1 expression in motor neurons by selective gene excision strongly slowed disease onset and early disease progression, whereas a similar lowering of mutant SOD1 expression within microglia strongly delayed disease progression after onset (4). Therefore, it is clear that multiple cell types in the spinal cord contribute to initial toxicity and disease progression.
One important question not addressed by these studies is whether the muscle itself also contributes to toxicity in ALS. One of the earliest findings in human ALS patients and in the mouse ALS model is the denervation of the neuromuscular junction. This denervation may result either from SOD1 mutant action within muscles that provokes muscle atrophy and that in turn actively damages the neurons, provoking synapse disruption, or from a damaged neuron retracting from the muscle (5, 6). Either way, the resulting disease progression leads to severe atrophy of skeletal muscles. Repetitive injection of myostatin antibody produced an increase in muscle mass and strength in SOD1G93A mice (7). Whether enhanced muscle mass per se can be beneficial remains untested, however, because in this report the effect on limb muscles was short lived, yielding neither a survival benefit nor preservation of muscle mass throughout disease progression.
In contrast, muscle hypertrophy induced by agents such as IGF-1 (8–10) or growth hormone (8–10) led to significant life extensions in ALS transgenic mice. For the IGF-1 studies, not only was there muscle hypertrophy, but also there was concomitant stimulation of muscle satellite cell proliferation and an increase of centrally nucleated muscle fibers, indicating regeneration (8). Added to these findings, a number of studies have shown that exercise is beneficial in ALS transgenic animals (11–13), with exercise and IGF-1 exhibiting a synergistic effect resulting in an increase in median life span by 83 days (11–13).
These prior efforts raised the likelihood that potential therapies that limited the synthesis of the disease-causing mutant SOD1 gene or that increased growth factor production might be acting in whole or in part on the muscle. Determining whether an initiating toxic insult or a determinant of disease progression after onset is developed within muscle is important, because if so, muscle is an attractive target for therapeutic development in ALS, especially by virus-mediated gene therapy approaches. Here we use three genetic approaches to test the role of muscle in mutant SOD1 disease onset and progression.
Results
Reducing Mutant SOD1 Within Muscle Does Not Affect Disease.
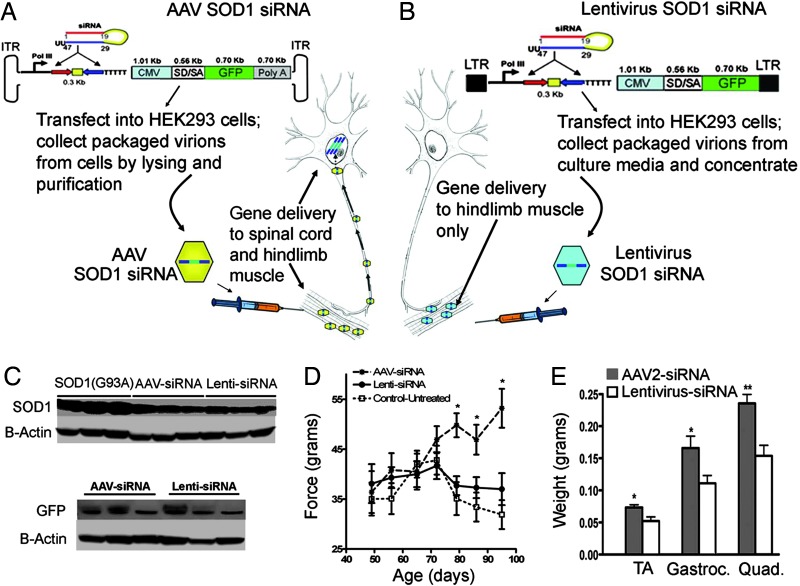
After intramuscular injection and its retrograde transport to spinal motor neurons (14), adeno-associated virus (AAV) encoding an siRNA against SOD1 (Fig. 1A) has previously been shown to maintain grip strength in SOD1G93A mice (10, 14, 15). To test whether SOD1G93A damage directly within muscle contributes to toxicity, a lentivirus was constructed that encodes a siRNA directed against SOD1. Because lentivirus pseudo-typed with vesicular stomatitus virus glycoprotein is not retrogradely transported (10, 16), intramuscular injection of this virus should produce siRNA exclusively within the muscles (Fig. 1B). Immunoblotting for SOD1 in extracts of gastrocnemius muscles were used to establish titers necessary to generate 60% suppression of mutant SOD1 after injection of lentivirus- or AAV-encoded siRNA to SOD1 (n = 10 each) (Fig. 1C). Parallel immunoblotting for GFP (encoded by a second gene carried by the lentivirus and AAV) verified that equivalent levels of siRNA were delivered to muscle by using the two different constructs (Fig. 1C).
Fig. 1.
Decreasing SOD1 in muscle does not improve grip strength in SOD1G93A mice. (A and B) Genes encoded in AAV are expressed in muscle and in motor neurons after retrograde transport. Genes encoded in lentivirus are expressed in muscle only. One hindlimb of 40-day-old SOD1G93A mice was injected with either lentivirus or AAV containing a siRNA directed against SOD1. The siRNA construct was identical in each case. (C) Protein blots of SOD1 and GFP levels in gastrocnemius from lentivirus- and AAV-injected SOD1G93A mice (three representative animals in each group). (D) Grip strength of the right treated hindlimb in AAV siRNA- vs. lentivirus siRNA-treated animals. (E) Wet weights of 98-day-old SOD1G93A mice treated with AAV siRNA vs. lentivirus siRNA (n = 10, average ± SE).
Hindlimb grip strength was recorded after injection of either viral construct. As previously demonstrated (15), animals injected with AAV-siRNA maintained grip strength between 75 and 95 days compared with untreated SOD1G93A mice that lost grip strength (Fig. 1D). Despite comparable suppression within muscle of mutant SOD1, animals injected with lentivirus-siRNA failed to maintain grip strength, similar to the performance of uninjected SOD1G93A littermates. Moreover, AAV siRNA-treated animals showed less muscle loss (age, 98 days) (Fig. 1E) than those injected with the lentivirus-siRNA, as expected from preservation of innervating motor neurons. Thus, substantially suppressing mutant SOD1 action selectively within muscles, even when initiated before disease onset, provided no benefit.
Reducing Mutant SOD1G37R Within Muscle by Selective Mutant Gene Excision Does Not Affect Onset or Survival.
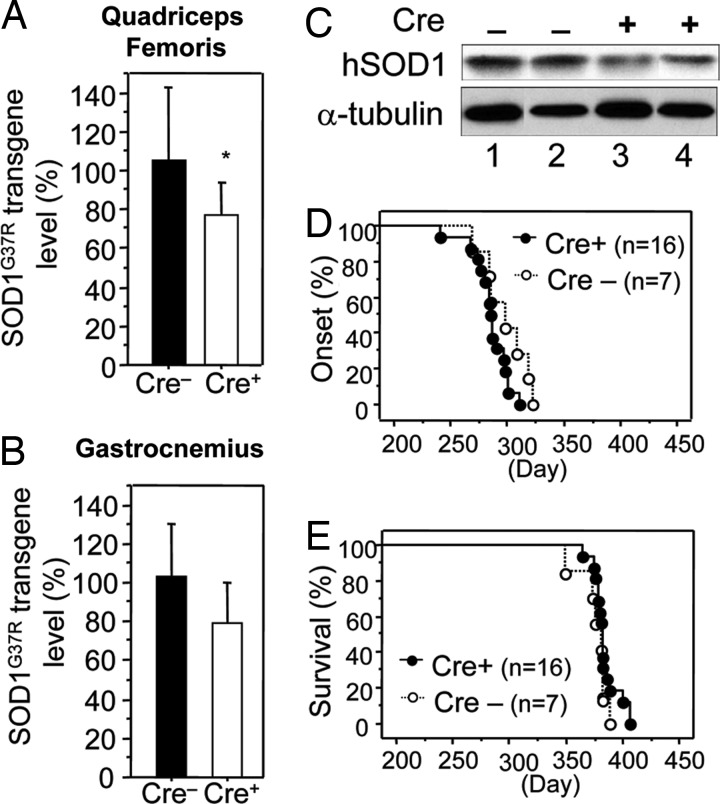
Mice heterozygous for a mutant human SOD1G37R gene flanked at both ends by 34-bp LoxP sites that allow excision by the Cre recombinase (4) were mated to mice carrying a Cre coding sequence under the control of the muscle creatine kinase (MCK) promoter that has previously been shown to be expressed exclusively in skeletal muscle (17). Quantitative PCR of genomic DNA derived from quadriceps femoris and gastrocnemius muscles from LoxSOD1G37R, MCK-Cre animals was used to identify a Cre-dependent, ≈25% reduction in SOD1G37R mRNA and protein (Fig. 2A–C). Muscle cell nuclei only account for ≈54% of the nuclei in muscle tissue, with endothelial, fibroblast, satellite, and other cell types providing the remaining 46% of nuclei (18). Because MCK-Cre only acts within muscle cell nuclei, the overall 25% decrease in SOD1 mRNA and protein in muscle tissue represents an ≈50% decrease in SOD1 mRNA and protein in the muscle fibers. Although a smaller degree of reduction in mutant SOD1 within microglia yields a striking 99-day extension of survival after disease onset (4), reduction in muscle had no effect on either onset (Cre−, 298 ± 20 days; Cre+, 285 ± 16 days) or survival (Cre−, 376 ± 13 days; Cre+, 384 ± 11 days) of LoxSOD1G37R mice (Fig. 2 D and E).
Fig. 2.
Reduction of mutant SOD1 from skeletal muscle does not affect disease onset or survival of LoxSOD1G37R mice. (A–C) LoxSOD1G37R transgene levels (A and B; Cre+, n = 4; Cre−, n = 3) in quadriceps femoris (A) and gastrocnemius (B) muscle and SOD1 protein levels in gastrocnemius muscle (C) from LoxSOD1G37R/MCK-Cre mice and LoxSOD1G37R mice were determined. Bars represent mean and standard deviation. ∗, P < 0.05; unpaired t test. For B, P = 0.11. (D and E) Onset (D) and survival (E) times of LoxSOD1G37R/MCK-Cre mice (Cre+) and littermate LoxSOD1G37R (Cre−) mice.
Follistatin-Induced Muscle Enhancement Is Not Protective in SOD1 Mutant-Mediated ALS.
Independent of SOD1 mutant damage directly within muscle, if it were true that muscle plays an active role in ALS, then enhancing muscle mass could be beneficial in SOD1G93A mice. To do this, muscles of SOD1G93A mice were injected with a viral construct encoding follistatin. AAV serotype 1 was chosen as the vector, given its ability to reliably and highly efficiently deliver transgenes to muscle (19, 20). Follistatin, originally described for its growth-enhancing effects on ovaries, is a negative regulator of myostatin (GDF8), a member of the TGF-β superfamily of growth and development factors. Follistatin overexpression has not shown any benefit or any harmful effects on motor neurons in previous studies (21). Inactivation of the myostatin gene or inhibition of myostatin protein with follistatin and other inhibitory proteins induces a hypermuscular phenotype in cattle and mice (22–25). Its effects on muscle mass have also been documented in other species. Myostatin's effects are thought to be mediated by inhibition of muscle cell proliferation, muscle hypertrophy, and DNA and protein synthesis (26, 27). In addition, myostatin inhibition reduces adipose tissue accumulation (28, 29).
Negative regulation of myostatin leads to enhanced muscle growth and is therefore being investigated in a number of muscular skeletal disorders, including muscular dystrophy (30, 31). In mouse models of these primary muscle diseases, inhibition of myostatin has dramatically improved the muscle phenotype (21, –36). Therefore, follistatin-mediated inhibition of myostatin would test whether enhancement of muscle mass and prevention of muscle atrophy might delay the motor functional decline in SOD1 mutant-mediated ALS.
AAV serotype 1 was constructed to encode human follistatin under the control of the strong human cytomegalovirus promoter (Fig. 3A). To test the ability of follistatin to reverse the myostatin-dependent inhibition of myoblast proliferation, C2C12 myoblasts were incubated with a growth inhibitory dose of myostatin in the presence or absence of conditioned media from AAV–follistatin-infected 293 cells or conditioned media from cells infected with AAV-encoding red fluorescent protein (RFP). Three days after myostatin inhibition, myoblast proliferation was assessed by measuring the conversion of yellow MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] to blue formazan, an assay that measures active mitochondrial reductase enzymes. Myoblasts cultured in growth media steadily increased in cell number compared with myostatin-treated cultures, in which a significant decrease in proliferation was seen. Follistatin-conditioned media reversed the inhibition of proliferation by myostatin, whereas control RFP-conditioned media did not (Fig. 3B), consistent with biologically active AAV-encoded follistatin.
Fig. 3.
Follistatin promotes myoblast proliferation in vitro. In vitro testing was performed to confirm the biological activity of follistatin to inhibit myostatin. (A) Schematic of follistatin in AAV vector and plasmids used for AAV production. CMV represents the cytomegalovirus promoter with a splice donor/acceptor sequence. Poly(A) represents the poly-adenylation sequence. The vector is flanked by the two AAV inverted terminal repeats from serotype 2. Rep2Cap1 represents the plasmid used for packaging the AAV into capsid serotype 1 along with the adenoviral helper plasmid. (B) Proliferation assay of myoblasts (C2C12) cultured in growth media in the presence or absence of myostatin (3 μg/ml) mixed with conditioned media from AAV-transduced cells expressing either RFP or follistatin (FS) (n = 4 average ± SE).
To directly assess whether increased muscle proliferation affected disease course in a mouse model of inherited ALS caused by mutation in SOD1, AAV–follistatin, or AAV–GFP (1 × 1011 viral genomes per injection) were injected bilaterally via intramuscular delivery into the hindlimb quadriceps and tibialis anterior muscles of 16 animals (age, 40 days; equal distribution of male and female). Both sets of mice reached end stage disease at ≈126 days. Despite this lack of difference in survival, follistatin-treated muscles showed gross changes, including widespread increased muscle mass, compared with the GFP-treated animals (Fig. 4A). Easily seen upon visual inspection (Fig. 4A), the wet weights of multiple muscles (n = 10–15 animals each), including the tibialis anterior, gastrocnemius, medial quadriceps, and triceps muscles, were significantly increased (P < 0.05) after injection of AAV–follistatin (Fig. 4B). Increased muscle mass was not limited to the hindlimb muscles injected (Fig. 4 A and B), illustrating a paracrine function for follistatin in affecting muscles at remote sites. By using an ELISA specific for human follistatin, circulating blood levels of follistatin were found to be significantly elevated (>10 ng/ml) at 100 days of age in the AAV–follistatin-treated animals but not in AAV–GFP animals (Fig. 4C).
Fig. 4.
Follistatin increases muscle mass in SOD1G93A mice. AAV–follistatin or AAV–GFP at 1 × 1011 viral genomes was injected into the hindlimbs (quadriceps and tibialis muscles) of SOD1G93A mice at 40 days of age (n = 15 per group). (A) Representative photograph of end-stage (126 days) SOD1G93A showing the widespread increase in muscle mass in AAV–follistatin-treated animals. (B) Wet weights of tibialis anterior, gastrocnemius, medial quadriceps, and triceps muscle from AAV–follistatin- and AAV–GFP-treated animals (n = 10–15 per group, average ± SE). (C) ELISA for follistatin in blood from AAV–GFP- and AAV–follistatin-treated animals (age, 100 day; n = 8 average ± SE).
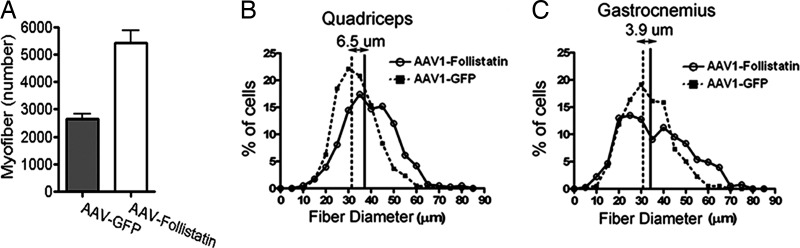
To determine whether the muscle weight increase was due to hyperplasia, hypertrophy, and/or muscle sparing in the ALS animals, myofiber numbers within the gastrocnemius muscle were counted in serial sections of AAV–follistatin- or AAV–GFP-treated groups (n = 8 animals). This analysis revealed that at end stage (126–127 days), approximately twice as many myofibers were present in the gastrocnemius of follistatin-treated animals compared with GFP-treated animals (n = 8 animals) (P < 0.01) (Fig. 5A). Follistatin-treated muscles also were slightly hypertrophic, based on measurements of myofiber diameters in the quadriceps and gastrocnemius muscles, compared with AAV–GFP-treated animals (Fig. 5 B and C).
Fig. 5.
Follistatin treatment increases the number and size of myofibers. (A) Myofibers were counted in serial sections of gastrocnemius and quadriceps muscle from AAV–follistatin- and AAV–GFP-treated SOD1G93A mice at end stage (126 days) (n = 8 per group, average ± SE). (B and C) Diameters of muscle fibers from quadriceps (B) and gastrocnemius (C) muscle in AAV–follistatin- and AAV–GFP-treated SOD1G93A mice at end stage (126 days) (n = 8 per group, average ± SE).
Increased muscle mass, increased myofiber number, and hypertrophy of muscle fibers translated, as expected, into increased strength in the follistatin-treated animals (Fig. 6A and B). By 75 days of age, follistatin-treated animals showed a 40% increase in hindlimb strength compared with GFP-treated littermates (Fig. 6A). Typically, nontransgenic mice continue to gain strength between 50 and 85 days, whereas the SOD1G93A mice fail to show the normal gain in strength at these ages and then lose strength as more neurons are lost (15). The follistatin-treated mutant SOD1 mice, however, continued to gain strength, remaining comparable with the nontransgenic animals at 75 days (data not shown). Strength in the forelimbs was also increased (Fig. 6B) from 65 days of age to end stage. The increased strength did not afford these mice better performance with other measures, including a rotarod test, presumably reflecting that strength is only one measure of performance in this particular test (Fig. 6C). Despite maintenance of muscle strength throughout most of disease progression and increased muscle mass even through end stage, no statistically significant increase in survival (defined by paralysis so severe that the animal was unable to right itself within 30 sec) was seen in the follistatin-treated mice versus untreated or AAV–GFP-treated cohorts (AAV–GFP, 126; AAV-follistatin, 130; P = 0.06; χ2 = 3.504) (Fig. 6D).
Fig. 6.
AAV–follistatin increases muscle strength but does not improve rotarod performance or significantly affect survival. (A and B) Grip strength of hindlimbs (A) and forelimbs (B) were recorded in AAV–GFP- and AAV–follistatin-treated SOD1G93A mice. (C) Latency to fall off the rotarod was measured in AAV–follistatin- AAV–GFP-treated SOD1G93A mice. (D) Survival analysis of AAV–follistatin- and AAV–GFP-treated SOD1G93A mice. AAV–GFP, 126 days; AAV–follistatin, 130 days; P = 0.06 (n = 15; litter-matched; the number of female mice equaled the number of male mice).
Discussion
An understanding of the role of muscle in ALS has practical implications for treating disease. Reduction in mutant SOD1 accumulation in muscle did not affect disease onset or progression. In contrast to its effects in motor neurons and microglia (4), these results clearly demonstrate that mutant SOD1 does not cause toxicity by its action within muscle. Thus, although multiple cell types contribute to a non-cell-autonomous death of motor neurons within the spinal cord, muscle is not one of the cell types that develops mutant-mediated damage that contributes directly to motor neuron loss. Furthermore, inhibition of myostatin with virally delivered follistatin produced a sustained enhancement of muscle mass and a prevention of muscle atrophy that were seen in a paracrine manner even at distal muscles even through end-stage disease. The fact that myostatin inhibition was able to increase strength even in diseased animals is encouraging for some limited, short-term, functional improvements using muscle-based therapies that may be considered in humans. However, follistatin-produced functional improvement of grip strength only for a short time and did not significantly affect survival in SOD1G93A mice. Thus, chronically enhanced muscle mass provides no long-term benefit in this example of inherited ALS. Finally, if muscle generated a negative or toxic signal to motor neurons, the increased muscle mass should have hastened onset and/or progression of disease. This result was not seen either.
An earlier report showed that exercise has beneficial effects in SOD1G93A animals, and a combination of exercise with IGF-1 treatment significantly prolongs survival in SOD1G93A animals (12). Although we cannot exclude that exercise has effects on muscle that are different from removing SOD1 by siRNA or enhancing muscle growth with follistatin treatment, our current data make it unlikely that the benefit is mediated by muscle alone. The salient effects of exercise in humans are loss of adipose tissue and increased muscle mass; however, there are likely to be more global effects. Exercise has been shown to induce gene changes in the spinal cord, such as the up-regulation of two specific antiapoptotic genes (12). Exercise-induced muscle strength has been documented to recruit motor units. Exercise may also induce trophic factor expression and secretion from the muscle, and the latter likely provides a benefit in delaying motor neuron death beyond simply enhancing muscle strength (37–39). Additionally, neural changes are seen in exercise, including the increased activation of motor neurons, increased motor synchronization, and cross-transference of strength between limbs (39–41). Endurance exercise has also been shown to induce significantly larger nerve terminals and results in increases in endplate potentials (42).
Despite all of these findings, no consensus has emerged from reports of the effects of exercise in ALS patients. Numerous reports have documented that strenuous exercise may have adverse effects; however, a number of reports have demonstrated beneficial effects of exercise in ALS (43–46). The type, intensity, and duration of exercise may all be influential components of the therapeutic efficacy of exercise, and the therapeutic targets of this exercise on motor neurons remain to be elucidated. Whatever these are, our results make it clear that any beneficial effect on muscles from exercise results from benefit to the motor neuron rather than merely increasing strength or mitigating mutant SOD1 action within muscle. Indeed, exercise may be promoting circulating factors to cross the blood–brain barrier (47, 48) as one mechanism for cellular protection in ALS.
IGF-1 has been demonstrated to be myotropic, to stimulate regeneration, and to be antiapoptotic in motor neurons when delivered intramuscularly by an AAV vector that undergoes retrograde transport and expression in motor neurons (10–13). IGF-1 has been shown to activate signal transduction mechanisms in a retrograde fashion (49). Production and secretion of an IGF-1 isoform solely in muscle delayed onset of disease by 10 days and prolonged survival by 30 days in SOD1G93A mice (8). These results were very similar to earlier gene therapy findings using AAV to elevate synthesis and secretion of the same IGF-1 isoform by motor neurons (8, 10). Although Dobrowolny et al. (8) concluded that the beneficial effects in an ALS mouse model derived from muscle-specific secretion of IGF-1 reflected muscle as the primary target for IGF-1, this conclusion relies on the assumption of extremely limited diffusion by attachment of this specific IGF-1 isoform to extracellular matrix components after its secretion. The proximity of the motor neuron synapse at the neuromuscular junction and the known retrograde signaling after uptake (50), however, make it all but certain that muscle-secreted IGF-1 also acts on the adjacent motor neurons. Thus, it seems most likely that synthesis by muscle of IGF-1 primarily benefits SOD1 mutant-mediated disease by affecting the innervating neuron at the neuromuscular junction, where IGF-1 can have profound effects.
Although muscle may be an attractive target for gene therapy approaches by forcing it to synthesize factors like IGF-1, whose delivery to spinal motor neurons can enhance motor neuron survival, it is clear, in view of our current evidence, that mutant SOD1 action directly within muscle is not a significant contributor to toxicity in ALS mice; neither can mutant SOD1's effects be alleviated by sustained increase in muscle mass, even when initiated before disease onset.
Experimental Procedures and Methods
Viral Vectors.
Human follistatin-344 was subcloned into the EcoRI site of Bluescript, containing a novel 5′ SfiI site and 3′ PmeI site. The cDNA was then subcloned directionally into these sites in a vector that was under the control of the human cytomegalovirus promoter and contained AAV serotype 2 inverted terminal repeats (51). The vector containing AAV serotype 2 siRNA SOD1 was produced as previously described (15). Recombinant AAV serotype 1 vectors were produced by triple transfection with calcium phosphate in HEK-293 cells, as previously described (52). Briefly, a plasmid containing the replication gene from serotype 2 and capsid gene from serotype 1 (52), along with a helper adenoviral plasmid (Stratagene, Palo Alto, CA), was used. Virus was collected 72 h after transfection and processed on cesium chloride gradients as previously described (51). A contract manufacturing company (Virapur, San Diego, CA) was used for some virus preparations and titers were confirmed. Titer was determined by quantitative PCR techniques, and titers were 3 × 1012 DNase-resistant particles per milliliter. Lentivirus pseudo-typed with vesicular stomatis virus glycoprotein was produced by quadruple transfection in HEK-293 cells, as previously described (53). Titers were determined to be 5 × 108 transducing units/ml based on GFP expression on serial dilution infections of HEK-293 cells.
In Vitro Assay.
C2C12 myoblasts were grown in DMEM (Invitrogen, Carlsbad, CA) containing 10% FBS (Invitrogen). Cell proliferation assays were conducted in 96-well microtiter plates (Nunc, Rochester, NY) and seeded at 1,000 cells per well. After attachment, myostatin (R & D Systems, Minneapolis, MN) was added at a concentration of 3 μg/ml in the presence or absence of conditioned media. Conditioned media were created by infecting a well of a 12-well dish with either 109 viral particles of AAV–RFP or AAV–follistatin and collecting the media 48 h after infection. A MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] proliferation assay was performed by using a commercially available kit (Pierce, Rockford, IL). Data were collected in quadruplicate and read on a microplate reader set for absorbance at 570 nM. Results were presented as means and standard errors.
Injection of Mice and Behavioral Testing.
A total of 1 × 1011 AAV viral particles were injected into both hindlimbs of SOD1G93A mice for follistatin studies or into one hindlimb for the siRNA studies. A total of 1 × 107 lentiviral particles were injected per hindlimb for lentiviral siRNA studies. Injections for follistatin studies were into the hindlimb quadriceps and tibialis muscles. Mice were observed daily for survival. Testing of motor function using a rotarod device (Columbus Instruments, Columbus, OH) began at 35–40 days of age. Each weekly session consisted of three trials on the elevated accelerating rotarod, beginning at 5 rpm. The time each mouse remained on the rod was registered. Grip strength measurements for forelimb and hindlimbs were tested weekly by using a grip strength meter (Columbus Instruments). Each weekly session consisted of four tests per animal per limb. For siRNA studies, grip strength meter testing was performed by allowing the animals to grasp a platform with one hindlimb (left or right) followed by pulling the animal until it released the platform; the force measurement was recorded in four separate trials. To determine mortality in a reliable and humane fashion, we used an artificial end point, defined by the inability of the mice to right themselves 30 sec after being placed on their sides. The moribund mice were scored as “dead” and were killed, and tissues were collected.
LoxSOD1G37R Mice.
Transgenic mice heterozygous for human SOD1G37R transgene flanked by loxP sequences (4) were mated to transgenic mice heterozygous for muscle-specific Cre recombinase (MCK-Cre) (17). Genotyping of LoxSOD1G37R and MCK-Cre transgenes was performed as described (4). Levels of LoxSODG37R in skeletal muscle were determined by quantitative PCR as described (4). Onset of disease in these animals was defined as the first sign of weight loss. Death was defined as above, as the inability to right themselves after 30 sec.
Muscle Fiber Number and Size.
Skeletal muscle hypertrophy and myofiber number were investigated. Muscles were embedded in OCT compound and snap-frozen in liquid-nitrogen-cooled isopentane. Transverse 10-μm sections were cut through the middle of the muscle, and sections were stained with H&E and trichrome stains. The sections (four sections each animal) were photographed on an Axiovert microscope connected to a micrometer (Zeiss, Thornwood, NY) on a Dell (Round Rock, TX) Workstation. The total area of the muscle cross-section was calculated, individual myofibers were counted, and diameters were measured. Graphs of total fiber numbers per section and a percentage of fiber diameters were plotted.
Immunoblotting and ELISA.
Muscles were immediately lysed in tissue protein extraction reagent (TPER; Pierce) and homogenized with a motorized homogenization device (Fisher Scientific, Waltham, MA). Protein was resolved on a 4–12% Bis-Tris SDS/PAGE system (Novex; Invitrogen) and transferred to nitrocellulose membranes (Invitrogen). Membranes were incubated with a 1:2,000 dilution of SOD1 antibody or a 1:5,000 dilution of β-actin antibody (Sigma, St. Louis, MO) or 1:3,000 α-tubulin antibody (Sigma) in 5% nonfat skim milk/0.1% Tween 20 in PBS, followed by incubation with horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG (Amersham Pharmacia Biosciences, Piscataway, NJ) and visualized with enzyme chemiluminescence (Amersham Pharmacia Biosciences). ELISAs were performed by using a commercially available ELISA against human follistatin (R & D Systems). Data were collected in triplicate for each animal and presented as means with standard error.
Statistical Analysis.
Survival analysis was performed by Kaplan–Meier analysis, which generates a χ2 value to test for significance. The Kaplan–Meier test was performed by using the log-rank test, equivalent to the Mantel–Haenszel test. In addition, two-tailed P values were calculated. When comparing survival curves, median survival times were calculated with a 95% confidence interval. All other statistical tests not involved in survival analysis were performed by multiway ANOVA followed by a Bonferroni post hoc analysis of means differences between groups (Prizm software; GraphPad, San Diego, CA).
Acknowledgments
We thank Lindsey Christian (Salk Institute) for molecular biology assistance, M. L. Gage for critical editorial comments, Dr. R. Kahn (Harvard Medical School, Boston, MA) for MCK-Cre mice, and Dr. S. Shimasaki (University of California at San Diego) for human follistatin plasmid. This work was supported by grants from the National Institute of Aging (to F.H.G.), National Institute of Neurological Disorders and Stroke (to B.K.K. and D.W.C.), Project ALS (to B.K.K. and F.H.G.), the Helping Link Foundation (to B.K.K.), and the ALS Association (to B.K.K., T.M.M., and D.W.C.). T.M.M. was supported by a K12 award from the National Institute of Aging. K.Y. was supported by a Career Development Award from the Muscular Dystrophy Association. D.W.C. receives salary support from the Ludwig Institute for Cancer Research.
Abbreviations
- ALS
amyotrophic lateral sclerosis
- AAV
adeno-associated virus
- SOD1
superoxide dismutase 1
- MCK
muscle creatine kinase
- RFP
red fluorescent protein.
Footnotes
The authors declare no conflict of interest.
References
- 1.Bruijn LI, Miller TM, Cleveland DW. Annu Rev Neurosci. 2004;27:723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- 2.Cleveland DW, Rothstein JD. Nat Rev Neurosci. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- 3.Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillee S, Rule M, McMahon AP, Doucette W, Siwek D, Ferrante RJ, et al. Science. 2003;302:113–117. doi: 10.1126/science.1086071. [DOI] [PubMed] [Google Scholar]
- 4.Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- 5.Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD. Exp Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Pun S, Santos AF, Saxena S, Xu L, Caroni P. Nat Neurosci. 2006;9:408–419. doi: 10.1038/nn1653. [DOI] [PubMed] [Google Scholar]
- 7.Holzbaur EL, Howland DS, Weber N, Wallace K, She Y, Kwak S, Tchistiakova LA, Murphy E, Hinson J, Karim R, et al. Neurobiol Dis. 2006;23:697–707. doi: 10.1016/j.nbd.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Dobrowolny G, Giacinti C, Pelosi L, Nicoletti C, Winn N, Barberi L, Molinaro M, Rosenthal N, Musaro A. J Cell Biol. 2005;168:193–199. doi: 10.1083/jcb.200407021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith RA, Melmed S, Sherman B, Frane J, Munsat TL, Festoff BW. Muscle Nerve. 1993;16:624–633. doi: 10.1002/mus.880160608. [DOI] [PubMed] [Google Scholar]
- 10.Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- 11.Kirkinezos IG, Hernandez D, Bradley WG, Moraes CT. Ann Neurol. 2003;53:804–807. doi: 10.1002/ana.10597. [DOI] [PubMed] [Google Scholar]
- 12.Kaspar BK, Frost LM, Christian L, Umapathi P, Gage FH. Ann Neurol. 2005;57:649–655. doi: 10.1002/ana.20451. [DOI] [PubMed] [Google Scholar]
- 13.Veldink JH, Bar PR, Joosten EA, Otten M, Wokke JH, van den Berg LH. Neuromuscular Disord. 2003;13:737–743. doi: 10.1016/s0960-8966(03)00104-4. [DOI] [PubMed] [Google Scholar]
- 14.Pirozzi M, Quattrini A, Andolfi G, Dina G, Malaguti MC, Auricchio A, Rugarli EI. J Clin Invest. 2006;116:202–208. doi: 10.1172/JCI26210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller TM, Kaspar BK, Kops GJ, Yamanaka K, Christian LJ, Gage FH, Cleveland DW. Ann Neurol. 2005;57:773–776. doi: 10.1002/ana.20453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazarakis ND, Azzouz M, Rohll JB, Ellard FM, Wilkes FJ, Olsen AL, Carter EE, Barber RD, Baban DF, Kingsman SM, et al. Hum Mol Genet. 2001;10:2109–2121. doi: 10.1093/hmg/10.19.2109. [DOI] [PubMed] [Google Scholar]
- 17.Bruning JC, Michael MD, Winnay JN, Hayashi T, Horsch D, Accili D, Goodyear LJ, Kahn CR. Mol Cell. 1998;2:559–569. doi: 10.1016/s1097-2765(00)80155-0. [DOI] [PubMed] [Google Scholar]
- 18.Schmalbruch H, Hellhammer U. Anat Rec. 1977;189:169–175. doi: 10.1002/ar.1091890204. [DOI] [PubMed] [Google Scholar]
- 19.Chao H, Liu Y, Rabinowitz J, Li C, Samulski RJ, Walsh CE. Mol Ther. 2000;2:619–623. doi: 10.1006/mthe.2000.0219. [DOI] [PubMed] [Google Scholar]
- 20.Xiao W, Chirmule N, Berta SC, McCullough B, Gao G, Wilson JM. J Virol. 1999;73:3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SJ, McPherron AC. Proc Natl Acad Sci USA. 2001;98:9306–9311. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grobet L, Martin LJ, Poncelet D, Pirottin D, Brouwers B, Riquet J, Schoeberlein A, Dunner S, Menissier F, Massabanda J, et al. Nat Genet. 1997;17:71–74. doi: 10.1038/ng0997-71. [DOI] [PubMed] [Google Scholar]
- 23.Kambadur R, Sharma M, Smith TP, Bass JJ. Genome Res. 1997;7:910–916. doi: 10.1101/gr.7.9.910. [DOI] [PubMed] [Google Scholar]
- 24.McPherron AC, Lee SJ. Proc Natl Acad Sci USA. 1997;94:12457–12461. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amthor H, Nicholas G, McKinnell I, Kemp CF, Sharma M, Kambadur R, Patel K. Dev Biol. 2004;270:19–30. doi: 10.1016/j.ydbio.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 26.McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. J Cell Biol. 2003;162:1135–1147. doi: 10.1083/jcb.200207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Girgenrath S, Song K, Whittemore LA. Muscle Nerve. 2005;31:34–40. doi: 10.1002/mus.20175. [DOI] [PubMed] [Google Scholar]
- 28.Thomas M, Langley B, Berry C, Sharma M, Kirk S, Bass J, Kambadur R. J Biol Chem. 2000;275:40235–40243. doi: 10.1074/jbc.M004356200. [DOI] [PubMed] [Google Scholar]
- 29.Lee SJ. Annu Rev Cell Dev Biol. 2004;20:61–86. doi: 10.1146/annurev.cellbio.20.012103.135836. [DOI] [PubMed] [Google Scholar]
- 30.Tobin JF, Celeste AJ. Curr Opin Pharmacol. 2005;5:328–332. doi: 10.1016/j.coph.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 31.Wagner KR. Curr Opin Rheumatol. 2005;17:720–724. doi: 10.1097/01.bor.0000184163.61558.ca. [DOI] [PubMed] [Google Scholar]
- 32.Wagner KR, McPherron AC, Winik N, Lee SJ. Ann Neurol. 2002;52:832–836. doi: 10.1002/ana.10385. [DOI] [PubMed] [Google Scholar]
- 33.Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS, Khurana TS. Nature. 2002;420:418–421. doi: 10.1038/nature01154. [DOI] [PubMed] [Google Scholar]
- 34.Engvall E, Wewer UM. FASEB J. 2003;17:1579–1584. doi: 10.1096/fj.02-1215rev. [DOI] [PubMed] [Google Scholar]
- 35.Wagner KR, Liu X, Chang X, Allen RE. Proc Natl Acad Sci USA. 2005;102:2519–2524. doi: 10.1073/pnas.0408729102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill JJ, Davies MV, Pearson AA, Wang JH, Hewick RM, Wolfman NM, Qiu Y. J Biol Chem. 2002;277:40735–40741. doi: 10.1074/jbc.M206379200. [DOI] [PubMed] [Google Scholar]
- 37.Funakoshi H, Belluardo N, Arenas E, Yamamoto Y, Casabona A, Persson H, Ibanez CF. Science. 1995;268:1495–1499. doi: 10.1126/science.7770776. [DOI] [PubMed] [Google Scholar]
- 38.Krivickas L. J Clin Neuromuscul Dis. 2003;5:29–39. doi: 10.1097/00131402-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Krivickas L. J Clin Neuromuscul Dis. 2003;5:29–39. doi: 10.1097/00131402-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Edstrom L, Grimby L. Muscle Nerve. 1986;9:104–126. doi: 10.1002/mus.880090203. [DOI] [PubMed] [Google Scholar]
- 41.Muller EA. Arch Phys Med Rehab. 1970;51:449–462. [PubMed] [Google Scholar]
- 42.Fahim MA. J Appl Physiol. 1997;83:59–66. doi: 10.1152/jappl.1997.83.1.59. [DOI] [PubMed] [Google Scholar]
- 43.Drory VE, Goltsman E, Reznik JG, Mosek A, Korczyn AD. J Neurol Sci. 2001;191:133–137. doi: 10.1016/s0022-510x(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 44.Bohannon RW. Phys Ther. 1983;63:965–968. doi: 10.1093/ptj/63.6.965. [DOI] [PubMed] [Google Scholar]
- 45.Pinto AC, Alves M, Nogueira A, Evangelista T, Carvalho J, Coelho A, de Carvalho M, Sales-Luis ML. J Neurol Sci. 1999;169:69–75. doi: 10.1016/s0022-510x(99)00218-x. [DOI] [PubMed] [Google Scholar]
- 46.Scarmeas N, Shih T, Stern Y, Ottman R, Rowland LP. Neurology. 2002;59:773–775. doi: 10.1212/wnl.59.5.773. [DOI] [PubMed] [Google Scholar]
- 47.Carro E, Trejo JL, Busiguina S, Torres-Aleman I. J Neurosci. 2001;21:5678–5684. doi: 10.1523/JNEUROSCI.21-15-05678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trejo JL, Carro E, Torres-Aleman I. J Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caroni P. Ann NY Acad Sci. 1993;692:209–222. doi: 10.1111/j.1749-6632.1993.tb26219.x. [DOI] [PubMed] [Google Scholar]
- 50.Rind HB, von Bartheld CS. Mol Cell Neurosci. 2002;19:58–71. doi: 10.1006/mcne.2001.1069. [DOI] [PubMed] [Google Scholar]
- 51.Kaspar BK, Erickson D, Schaffer D, Hinh L, Gage FH, Peterson DA. Mol Ther. 2002;5:50–56. doi: 10.1006/mthe.2001.0520. [DOI] [PubMed] [Google Scholar]
- 52.Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X, Samulski RJ. J Virol. 2002;76:791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kafri T, Blomer U, Peterson DA, Gage FH, Verma IM. Nat Genet. 1997;17:314–317. doi: 10.1038/ng1197-314. [DOI] [PubMed] [Google Scholar]