Abstract
The kidney plays a key role in maintaining potassium (K) homeostasis. K excretion is determined by the balance between K secretion and absorption in distal tubule segments such as the connecting tubule and cortical collecting duct. K secretion takes place by K entering principal cells (PC) from blood side through Na+, K+-ATPase and being secreted into the lumen via both ROMK-like small-conductance K (SK) channels and Ca2+-activated big-conductance K (BK) channels. K reabsorption occurs by stimulation of apical K/H-ATPase and inhibition of K recycling across the apical membrane in intercalated cells (IC). The role of ROMK channels in K secretion is well documented. However, the importance of BK channels in mediating K secretion is incompletely understood. It has been shown that their activity increases with high tubule flow rate and augmented K intake. However, BK channels have a low open probability and are mainly located in IC, which lack appropriate transporters for effective K secretion. Here we demonstrate that inhibition of ERK and P38 MAPKs stimulates BK channels in both PC and IC in the cortical collecting duct and that changes in K intake modulate their activity. Under control conditions, BK channel activity in PC was low but increased significantly by inhibition of both ERK and P38. Blocking MAPKs also increased channel open probability of BK in IC and thereby it may affect K backflux and net K absorption Thus, modulation of ERK and P38 MAPK activity is involved in controlling net K secretion in the distal nephron.
Keywords: ERK MAPK, K absorption, K secretion, P38 MAPK, ROMK
The kidney plays a key role in maintaining external potassium (K) balance by responding effectively to changes in dietary K intake. It has been well established that high K intake increases K secretion in principal cells (PC) and low K intake suppresses K secretion in PC and enhances K reabsorption in intercalated cells (IC) (1). K secretion takes place by K entering PC through Na+, K+-ATPase followed by passive exit into the lumen through apical K channels. K reabsorption involves apical uptake of K into IC by K/H-ATPase. The effectiveness of this process depends critically upon K channel activity: increased apical K conductance diminishes and decreased K channel activity enhances K absorption in IC.
Patch-clamp studies and molecular cloning have demonstrated that ROMK-like small-conductance K (SK) channels (2–4) and big-conductance K (BK) channels (5–8) are the two main types of K channels in the apical membrane of the cortical collecting duct (CCD). It is generally accepted that ROMK channels are responsible for K secretion under conditions of normal K intake because these channels have high open probability and expression (9). In contrast, BK channels have a low open probability and low channel density (6, 7). Thus, it is unlikely that BK channels play a major role in K secretion under normal conditions. However, several studies have shown that BK channel activity increased when tubule flow rate was high or dietary K intake elevated (10–12). However, BK channels are mainly expressed in the apical membrane of IC, which have low Na+, K+-ATPase activity (6, 13). In the absence of such basolateral K-uptake mechanisms, it is unresolved how K secretion through BK channels could take place in IC. On the other hand, the number of BK channels in the apical membrane of PC is quite low and may thus not account for a significant K secretion. In the present study, we have addressed this problem and demonstrated that BK channel activity in the PC and IC is normally inhibited by P38 MAPK and ERK and that high K intake suppresses MAPK activity and increases BK channels.
Results
Biophysical Properties of BK.
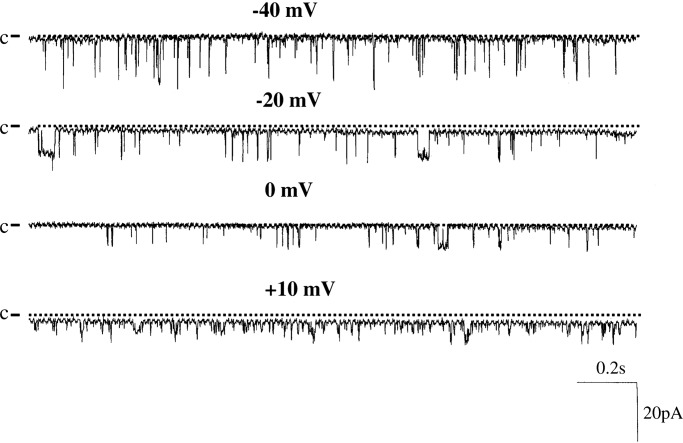
Fig. 1 is a representative channel recording showing BK channel activity in a cell-attached patch of PC. We confirmed previous findings that the probability of finding BK channels in PC was low (14) and observed channel activity in only 4 out of 50 patches (8%) in cell-attached patches. The mean BK channel activity (NPo) of four patches was 0.01 ± 0.004. We also studied BK channels in IC and confirmed that BK channel activity was more often observed in IC than in PC (14). We detected BK channels in cell-attached patches in 7 out of 25 patches (28%), and their NPo was low (0.02 ± 0.005). Thus, our data confirm that under physiological conditions, BK channel activity in IC exceeded that in PC.
Fig. 1.
A single channel recording showing the BK channel activity in a cell-attached patch of PC. The channel closed level is indicated by C and a dotted line, and the pipette holding potential (negative values indicate hyperpolarization) is indicated at the top of each trace.
We also measured the channel conductance of cell-attached and in inside-out patches. The channel conductance of cell-attached patches at 0 mV holding potential (spontaneous cell membrane potential) with 140 mM KCl in the pipette and 140 mM NaCl (containing 5 mM KCl) in the bath was 130 ± 10 pS. Channel conductance increased to 170 ± 10 pS with symmetrical 140 mM KCl solutions.
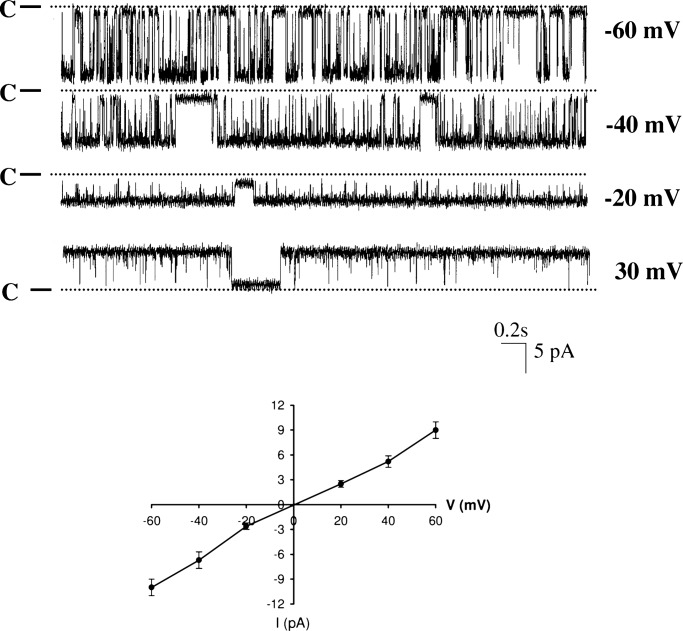
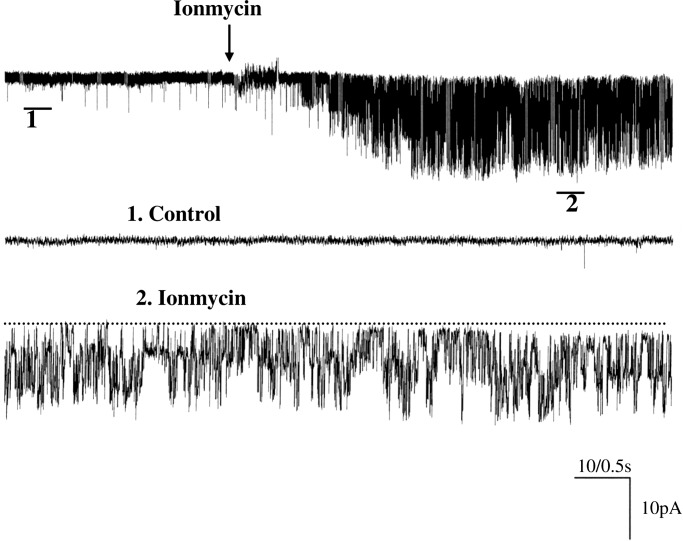
Fig. 2 is a representative channel recording with symmetrical 140 mM KCl in both bath and pipette in an inside-out patch in PC. To activate BK channel activity, 10 μM Ca2+ was added to the cytosolic bath solution. From inspection of Fig. 2, it is apparent that BK channels were activated by 10 μM Ca2+. In contrast, removal of Ca2+ completely inactivated BK channels (data not shown). To determine whether there is a pool of silent BK channels in PC, we used ionmycin (1 μM), an agent known to raise intracellular Ca2+ in CCD (15). Indeed, application of ionmycin (1 μM) in cell-attached patches increased the probability of finding BK channels in PC, and we observed BK channels in 3 out of 12 patches (25%) and increased NPo from 0.01 ± 0.004 to 0.9 ± 0.17 (Fig. 3) (n = 3). This suggests that under physiological conditions, BK channel activity was inhibited in PC but that the number of potentially available BK channels in the apical membrane of PC may increase with appropriate stimulation. However, the intracellular Ca2+ concentration raised by ionmycin should be much higher than that induced by physiological stimulation such as high flow rate. Thus, the mechanism by which high flow rate stimulates BK channels may be different from that with ionmycin.
Fig. 2.
A single channel recording showing the BK channel activity in an inside-out patch of PC with symmetrical 140 mM KCl solution. The channel closed level is indicated by C and a dotted line, and the pipette holding potential (negative values indicate hyperpolarization) is indicated at the right end of each trace. The I–V (current–voltage) curve of BK channels in inside-out patches with symmetrical 140 mM KCl in bath and pipette is shown at the bottom of the figure.
Fig. 3.
A channel recording showing the effect of ionmycin on BK channels in a cell-attached patch of PC. The top trace shows the time course of the experiment, and the two parts of the data indicated by numbers are demonstrated in a fast-time resolution.
Effects of ERK and P38 MAPK on BK Channels.
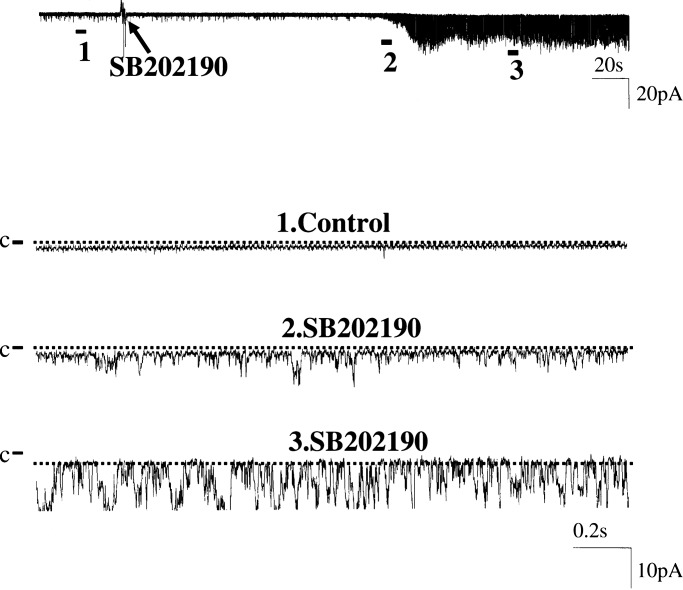
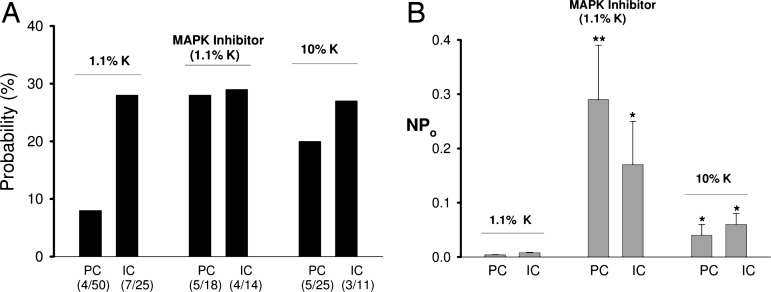
We also examined the effect of ERK and P38 MAPKs on BK channels because a previous study had shown that both kinase activities were closely related to dietary changes of K intake (16). We first investigated the effect of inhibiting P38 on BK channels in cell-attached patches of PC. Fig. 4 is a recording showing the effect of SB202190 (5 μM), a specific inhibitor of P38 (17). Before inhibitor application, BK channel activity was insignificant. Blockade of P38 increased NPo to 0.6. We observed a positive response of BK channel activity to SB202190 in 3 out of a total of 15 patches. This response is significantly higher than those before inhibition of P38 (4 out of 50 patches have BK channels). Mean NPo increased significantly from 0.004 ± 0.001 to 0.12 ± 0.06 (n = 15, P < 0.05). However, application of PD098059 (50 μM), an agent which blocks ERK activation (17), had no significant effect on SK channels because it stimulated BK channel activity in only 1 out of 11 patches (data not shown). However, suppressing both ERK and P38 MAPKs had an additive effect on BK channel activity. As demonstrated in Fig. 5A, simultaneous inhibition of ERK and P38 increased the probability of finding BK channels from 8 to 28% (5 out of 18 patches).
Fig. 4.
A channel recording showing the effect of inhibiting P38 MAPK with SB202190 on BK channels in a cell-attached patch of PC. The top trace shows the time course of the experiment, and the three parts of the data indicated by numbers are demonstrated in a fast-time resolution. The channel closed level is indicated by C and a dotted line.
Fig. 5.
A bar graph shows the probability of finding BK channels identified in PC and IC (A) and channel activity (NPo) (B) in the absence (left set of bars) or presence (middle set of bars) of PD098059+SB202190 or in the CCD from rats on a high K diet (right set of bars). The number shown below the bar indicates the positive patch (with BK channel) (top number) and total patches (bottom number), respectively. ∗∗, P < 0.02 between control (1.1% K) groups; ∗, P < 0.05 between experimental groups.
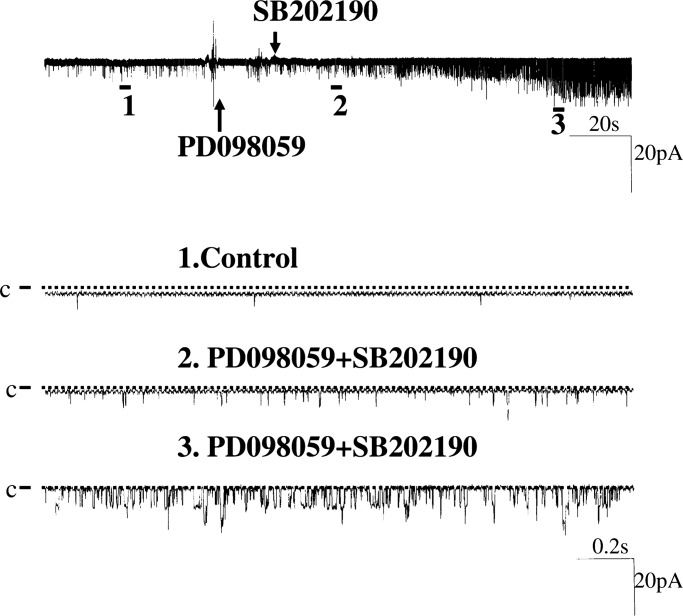
Fig. 6 is a channel recording showing the stimulating effect of PD098059 and SB202190 on BK channel activity. Although application of PD098059 alone did not increase BK channel activity immediately, BK channel activity is stimulated significantly after application of SB202190 in the presence of PD098059. Data summarized in Fig. 5B demonstrated that inhibition of both ERK and P38 significantly increased NPo from 0.004 ± 0.001 to 0.29 ± 0.10 (n = 18, P < 0.02). We also investigated the effect of inhibiting MAPK on BK channels in IC. Results summarized in Fig. 5A show that the BK channel activity was observed in 4 out of 14 patches (29%) in the presence of PD098059 and SB202190. This value is not different from control conditions. However, inhibition of MAPK significantly increased the open probability of BK channels in IC from 0.008 ± 0.001 to 0.17 ± 0.08 (n = 14, P < 0.05; Fig. 5B).
Fig. 6.
A channel recording showing the effect of inhibiting ERK with PD 098059 and P38 MAPK with SB202190 on BK channels in a cell-attached patch of PC. The top trace shows the time course of the experiment, and the three parts of the data indicated by numbers are demonstrated in a fast-time resolution.
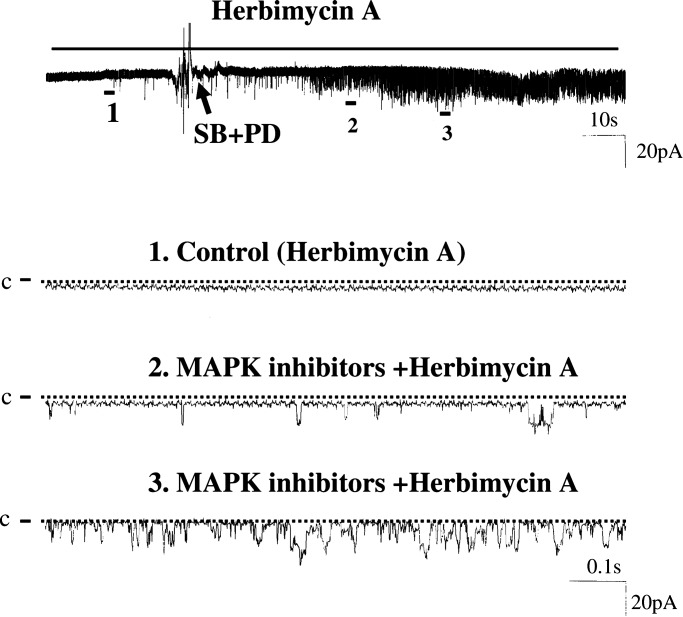
Because MAPK has been shown to regulate the activity of protein tyrosine kinase (PTK) (16), which has been shown to regulate apical ROMK in the CCD (18), we next examined whether the inhibitory effects of ERK and P38 on BK channels requires the involvement of PTK. Thus, we treated isolated CCDs with 1 μM herbimycin A to inhibit PTK and examined the effect of PD098059 and SB202190 (Fig. 7). In the presence of herbimycin A, we observed that inhibition of ERK and P38 increased BK channel activity in PC in 3 out of 10 patches and raised mean NPo from 0.004 ± 0.001 to 0.3 ± 0.15 (n = 10, P < 0.05). Thus, PTK was not involved in mediating the stimulatory effect of PD098059 and SB202190 on BK channels.
Fig. 7.
A channel recording showing the effect of inhibiting ERK and P38 MAPK on BK channels in a cell-attached patch of PC in the presence of herbimycin A. The top trace shows the time course of the experiment, and the three parts of the data indicated by numbers are demonstrated in a fast-time resolution.
Effect of High K Intake on MAPK Phosphorylation and BK Channels.
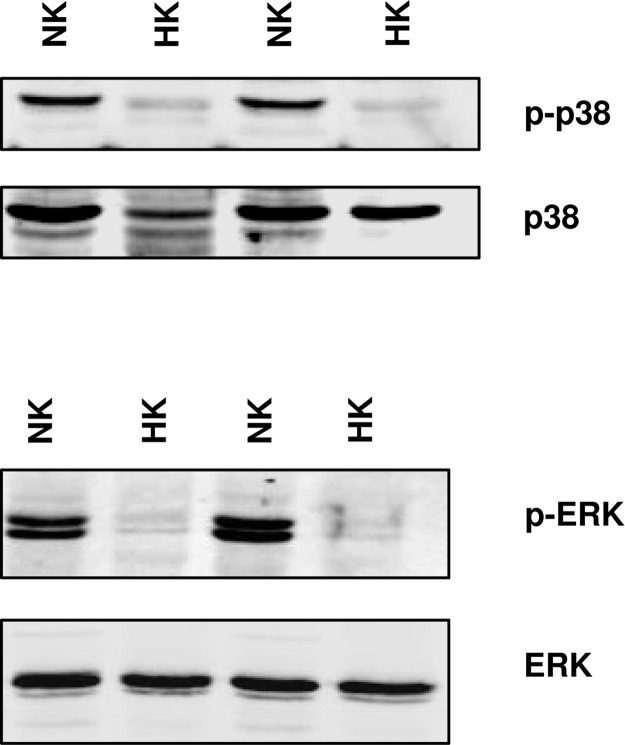
After demonstrating that inhibition of P38 and ERK stimulates BK channels, we examined BK channel activity in the CCD from rats on a high K diet for 7 days. Although previous study had failed to observe a change of BK channel number in PC in response to a high K intake, our previous experiments were performed with high KCl bath solutions (19). Because BK channels are known to be voltage-sensitive, high KCl-induced depolarization of the cell membrane potential would be expected to deactivate BK channels. In the present study, we examined BK channel activity in the CCD incubated in a NaCl solution with only 5 mM K. Results summarized in Fig. 5A show that high K intake increases the channel number in patches of PC (5 out of 25). However, the probability of finding BK channels in IC remained unchanged (3 out of 11). Channel open probability of BK increased slightly in both PC (0.04 ± 0.02) and IC (0.05 ± 0.02). We conclude that inhibition of P38 and ERK stimulates BK channel activity in both PC and IC. However, it only increases the BK channel number in PC. We also examined the effect of high K intake on the phosphorylation of P38 and ERK in the renal cortex and outer medulla. Fig. 8 is a representative example of a Western blot showing that high K intake significantly decreases the phosphorylation of ERK and P38 MAPK by 50 ± 5% (n = 3 rats). Thus, our results show that high K intake increases the BK channel activity and decreases the phosphorylation of MAPKs.
Fig. 8.
A Western blot showing the effect of K intake on the phosphorylation of ERK and P38 MAPK and the total MAPK in renal cortex and outer medulla.
Discussion
The main findings of the present study are that BK channels are suppressed by ERK and P38 MAPK in the CCD under control conditions (normal K diet), that their inhibition increases BK channel activity in both PC and IC, and that changes in MAPK kinase activity are involved in the kidney's response to alterations in K metabolism. The CCD is responsible for the hormone-regulated Na absorption and K secretion (20, 21). Secretion of K involves K entry across the basolateral membrane through Na+, K+-ATPase followed by K exit across the apical membrane via apical K channels (1). Because of their high open probability and channel density, SK channels (ROMK) are thought to be largely responsible for K secretion in the connecting tubule and CCD under physiological conditions. SK channels are sensitive to cytosolic changes in pH (22, 23) and regulated by protein kinase A (24, 25), protein kinase C (26), and protein tyrosine kinase. The latter is involved in mediating the effect of K restriction on SK channels (27). Changes in pH and K intake are well known to regulate renal K secretion and K channel activity (28).
In contrast to SK, BK channels in the connecting tubule and CCD have low channel open probability and numbers under normal conditions. Thus, it is believed that they do not play a significant role in K secretion under normal conditions and that their renal functions are limited to cell volume regulation (29) and mesangial cell contraction (30). This view is also supported by observations that inhibition of the Ca2+-activated BK channel does not affect renal K excretion in distal nephron in C57/Bl-6 mice fed a normal (1%) K diet (31). Perfusing isolated rabbit CCD at low physiological flow rate also provided no evidence for participation of BK channels in K secretion (11). However, recent evidence suggests that BK channels are involved in K secretion under certain circumstances. Thus, at high flow rates, inhibition of BK channels suppresses the transepithelial potential and K secretion in the isolated rabbit CCD (11). Moreover, micropuncture studies in mice demonstrated that iberiotoxin, a specific inhibitor of Ca2+-activated BK channels, decreased K secretion in the distal nephron in mice on a high K diet (31). Therefore, it is likely that the Ca2+-activated BK channels are involved in renal K secretion when tubule flow rate is high or dietary K intake increases.
However, two unresolved problems remain for understanding the mechanism by which BK channels participate in K secretion: their low open probability and location. Because BK channels have low channel open probability at physiological cell Ca2+ concentrations and the normal cell membrane potentials, it is unlikely that they could account for a significant K secretion. However, their contribution to secretion could become significant at high tubule flow rates, which increase the intracellular Ca2+ concentration into a range known to stimulate BK channels (32). The second problem of BK in K secretion is their preferential localization in IC as observed in patch-clamp experiments (6), although immunolocalization studies have reported that BK channels were expressed in PC (33). However, a separate study observed a strong staining of BK α subunit in the apical membrane of IC of rabbit CCD (34). The present patch-clamp data confirmed that 80% of BK channels are located in the apical membrane of IC under control conditions. Although the number of potentially active BK channels in PC is low, it may be underestimated because BK channels are constitutively blocked. This notion is supported by our observation that inhibition of P38 and ERK increased the number of BK channels in PC to the same extent as that in IC, suggesting that K secretion through BK channels could take place in PC. Using the technical parameters presented by Gray et al. (35) (apical surface area, 185 μM2; pipette tip size, 1 μM2), the calculated total BK channel conductance per cell is 1.95 nS [0.28 (probability) × 0.29 (NPo) × 185 (area) × 130 pS] after inhibition of P38 and ERK. This value is similar to the value of calculated ROMK channel conductance per PC (1.3 nS) (35). Thus, we hypothesize that BK channels in PC are suppressed under physiological conditions but increase with inhibition of P38 and ERK and could significantly contribute to K secretion.
There are at least four types of MAPK (36) including ERK1/2, JNK, and P38, which have been expressed in the kidney. The relatively fast onset of MAPK inhibition makes it unlikely that their effects require nuclear translocation and gene translation. Channel phosphorylation by MAPK has been shown to regulate a variety of K channels (37–39) and ENaC (40). However, it remains unresolved whether MAPK-induced inhibition of BK channel is the result of direct phosphorylation of the BK channel protein or due to interaction with associated proteins of BK channels.
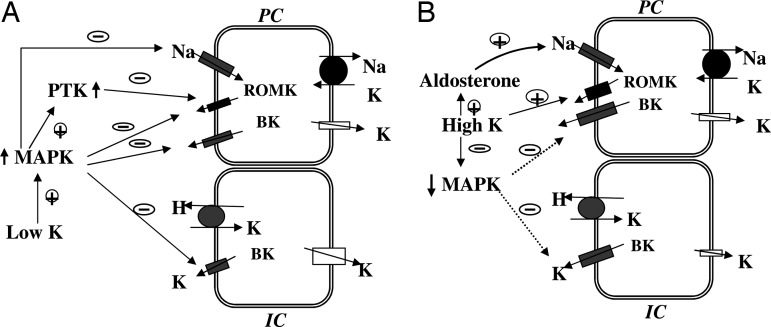
Fig. 9A is a model illustrating the role of MAPK in the suppression of K secretion in the CCD. Low K intake stimulates the activity of MAPKs, which not only inhibits ROMK and BK channels but also ENaC. MAPK inhibits ENaC activity by phosphorylation of the ENaC β and γ subunits and enhances the interaction between ENaC and ubiquitin ligase Nedd4 (40). This interaction should decrease ENaC expression and, accordingly, the driving force for K exit across the apical membrane.
We have previously demonstrated that low K intake stimulates the phosphorylation of P38 and ERK in the kidney (41), and in the present study we show that high K intake suppresses the phosphorylation of P38 and ERK. Such inhibition of MAPK phosphorylation may be responsible for increasing the channel number and channel open probability of BK channels in the CCD from rats on a high K diet (Fig. 9B). A high K intake may also increase flow rate. A high flow rate has been shown to increase intracellular Ca2+ concentration (32), which not only stimulates BK channels directly but also may activate calcineurin, a phosphatase which has been reported to inactivate P38 MAPK (42). Moreover, high K intake elevates aldosterone, which stimulates Na absorption and affects apical membrane depolarization, which further activates BK channels. The role of BK channels during adaptation to a high K diet has been also suggested by studies in which inhibition of BK channels decreased K secretion in the distal nephron from high K-adapted animals (31).
Fig. 9.
A cell model illustrating the role of MAPK in the regulation of K secretion and K absorption during K restriction (A) and high K intake (B). The dotted line indicates a diminished effect.
The regulation of BK channels by MAPK may also play an important role in K absorption in the CCD and outer medullary collecting duct (Fig. 9A). Low K intake stimulates net K absorption by activation of H/K-ATPase and inhibition of apical K channels in IC (43, 44). BK channels in IC may serve as a K recycling route across the apical membrane such that the efficiency of reabsorption in K depletion increased as MAPK suppresses BK channel activity. We speculate that MAPK may also stimulate K/H-ATPase and be responsible for increased K absorption induced by dietary K restriction. In contrast, when dietary K intake is normal or high, a low MAPK activity permits K recycling, and K taken by apical H/K-ATPase diffuses back into the lumen. We conclude that MAPK may play a role not only in the regulation of K secretion but also of K reabsorption through their effects on BK channels in the CCD.
Materials and Methods
Preparation of CCDs.
Pathogen-free Sprague–Dawley rats of both sexes (50–60 g) were purchased from Taconic Farms, Inc. (Germantown, NY). Animals were maintained on a normal (1.1%, wt/wt) or high (10%) K diet (Harlan Teklad, Madison, WI) and had free access to water for 1 week. Rats were killed by cervical dislocation, and kidneys were removed immediately for further isolation of CCDs. Several thin slices (<1 mm) were cut with a razor blade and placed in ice-cold Ringer's solution until isolation of tubules. The dissection was carried out at room temperature, and two watchmaker forceps were used to isolate the single CCD. The tubules were then immobilized by placing them on a 5 × 5 mm cover glass coated with polylysine (Sigma, St. Louis, MO). The cover glass was transferred to a chamber (1,000 μl) mounted on an inverted Nikon microscope (Nikon, Tokyo, Japan). The tubules were superfused with Hepes-buffered NaCl solution, and the temperature of the chamber was maintained at 37 ± 1°C by circulating warm water surrounding the chamber. To gain access to the apical membrane, a sharp pipette was used to cut the tubules.
Patch-Clamp Technique.
The patch pipettes were drawn with a PP-81 puller (Narishige, Greenvale, NY) and polished with a home-made fire-polisher. The currents were recorded with an Axon200A patch-clamp amplifier (Axon Instruments, Burlingame, CA) and low-pass filtered at 1 KHz by using an eight-pole Bessel filter (902LPF; Frequency Devices, Haverhill, MA). Data were digitized by Axon interface (Digidata1200) and stored on the hard drive of an IBM-compatible Pentium computer (Gateway 2000; Gateway, Irvine, CA). We used the pClamp software system 6.04 (Axon Instruments) to analyze the data. Channel activity was defined as NPo that was calculated from data samples of 60-second duration in the steady state as follows: NPo = Σ(t1 + t2 + … ti), where ti is the fractional open time spent at each of the observed current levels. The channel conductance was determined by measuring the current at different holding potentials. Both PC and IC were selected for patch-clamp analysis. The PC cells were recognized by their typical hexagonal shape and large flat surface. This characterization has been used by many investigators and confirmed by the presence of ROMK and ENaC in the apical membrane (6, 45, 46). Morphological characters of IC were: (i) their irregular shape; and (ii) a surface area higher than those of PC under Hoffman optics. After forming high-resistance seals, we determined the presence of BK channels by a brief 60-mV depolarization (10 seconds) followed by a 60-mV repolarization.
Western Blotting.
Proteins, which were homogenized from renal cortex and outer medulla, were separated by electrophoresis on 8–10% SDS-polyacrylamide gels and transferred to Immuno-Blot PVDF membrane (Bio-Rad, Hercules, CA). The membrane was blocked with Odyssey blocking buffer and incubated with the primary antibody at 4°C for 12 h. The membrane was washed four times for 5 min with PBS containing 0.1% Tween 20 and followed by incubation with the secondary antibody for additional 30 min. The membrane was then washed several times and scanned by Odyssey infrared imaging system (LI-COR, Lincoln, NE) at wavelength of 680 nm.
Experimental Solution and Statistics.
Antibodies to Phospho-P38, P38, Phospho-ERK, and ERK were purchased from Santa Cruz (Santa Cruz, CA).The pipette solution contained (in mM) 140 KCl, 1.8 MgCl2, and 10 Hepes (pH 7.4). The bath solution for cell-attached patches was composed of (in mM) 140 NaCl, 5 KCl, 1.8 CaCl2, 1.8 MgCl2, and 10 Hepes (pH 7.4). For inside-out patches, the bath solution was composed of 140 mM KCl, 10 μM CaCl2, 1.8 mM MgCl2, and 10 mM Hepes (pH 7.4). Ionmycin was purchased from Sigma, whereas SB202190, PD098059, and herbimycin A were obtained from Biomol (Plymouth Meeting, PA). Data are shown as mean ± SEM, and a paired Student t test was used to calculate the significance between the control and experimental groups. Statistical significance was taken as P < 0.05.
Acknowledgments
This work was supported by National Institutes of Health Grants DK54983 and DK47402.
Abbreviations
- K
potassium
- SK
small-conductance K
- BK
big-conductance K
- PC
principal cells
- IC
intercalated cells
- CCD
cortical collecting duct.
Footnotes
The authors declare no conflict of interest.
References
- 1.Giebisch G. Am J Physiol. 1998;274:F817–F833. doi: 10.1152/ajprenal.1998.274.5.F817. [DOI] [PubMed] [Google Scholar]
- 2.Ho K, Nichols CG, Lederer WJ, Lytton J, Vassilev PM, Kanazirska MV, Hebert SC. Nature. 1993;362:31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- 3.Wang W, Hebert SC, Giebisch G. Ann Rev Physiol. 1997;59:413–436. doi: 10.1146/annurev.physiol.59.1.413. [DOI] [PubMed] [Google Scholar]
- 4.Frindt G, Palmer LG. Am J Physiol. 1989;256:F143–F151. doi: 10.1152/ajprenal.1989.256.1.F143. [DOI] [PubMed] [Google Scholar]
- 5.Morita T, Hanaoka K, Morales MM, Montrose-Rafizadeh C, Guggino WB. Am J Physiol. 1997;273:F615–F624. doi: 10.1152/ajprenal.1997.273.4.F615. [DOI] [PubMed] [Google Scholar]
- 6.Frindt G, Palmer LG. Am J Physiol. 1987;252:F458–F467. doi: 10.1152/ajprenal.1987.252.3.F458. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch J, Leipziger J, Frobe U, Schlatter E. Pflugers Arch Eur J Physiol. 1993;422:492–498. doi: 10.1007/BF00375077. [DOI] [PubMed] [Google Scholar]
- 8.Schlatter E, Bleich M, Hirsch J, Markstahler U, Frobe U, Greger R. Pflugers Arch Eur J Physiol. 1993;422:481–491. doi: 10.1007/BF00375076. [DOI] [PubMed] [Google Scholar]
- 9.Hebert SC, Desir G, Giebisch G, Wang W. Physiol Rev. 2005;85:319–371. doi: 10.1152/physrev.00051.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woda CB, Miyawaki N, Ramalakshmi S, Ramkumar M, Rojas R, Zavilowitz B, Kleyman TR, Satlin LM. Am J Physiol. 2003;285:F629–F639. doi: 10.1152/ajprenal.00191.2003. [DOI] [PubMed] [Google Scholar]
- 11.Woda CB, Bragin A, Kleyman TR, Satlin LM. Am J Physiol. 2001;280:F786–F793. doi: 10.1152/ajprenal.2001.280.5.F786. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi J, Imai M. J Membr Biol. 1998;164:35–45. doi: 10.1007/s002329900391. [DOI] [PubMed] [Google Scholar]
- 13.Beck FX, Dorge A, Blumner E, Giebisch G, Thurau K. Kidney Int. 1988;33:642–651. doi: 10.1038/ki.1988.47. [DOI] [PubMed] [Google Scholar]
- 14.Frindt G, Palmer LG. Am J Physiol. 1987;252:F458–F467. doi: 10.1152/ajprenal.1987.252.3.F458. [DOI] [PubMed] [Google Scholar]
- 15.Wei Y, Wang WH. Am J Physiol. 2001;280:C920–C928. doi: 10.1152/ajpcell.2001.280.4.C920. [DOI] [PubMed] [Google Scholar]
- 16.Babilonia E, Li D, Wang ZJ, Sun P, Lin DH, Wang WH. J Am Soc Nephrol. 2006;17:2687–2696. doi: 10.1681/ASN.2006050426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 18.Wei Y, Bloom P, Lin DH, Gu RM, Wang WH. Am J Physiol. 2001;281:F206–F212. doi: 10.1152/ajprenal.2001.281.2.F206. [DOI] [PubMed] [Google Scholar]
- 19.Wang W, Schwab A, Giebisch G. Am J Physiol. 1990;259:F494–F502. doi: 10.1152/ajprenal.1990.259.3.F494. [DOI] [PubMed] [Google Scholar]
- 20.Palmer LG. Am J Physiol. 1999;277:F821–F825. doi: 10.1152/ajprenal.1999.277.6.F821. [DOI] [PubMed] [Google Scholar]
- 21.Garty H, Palmer LG. Physiol Rev. 1997;77:359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- 22.Sackin H, Nanazashvili M, Palmer LG, Krambis M, Walters DE. Biophys J. 2005;88:2597–2606. doi: 10.1529/biophysj.104.051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fakler B, Schultz JH, Yang J, Schulte U, Brändle U, Zenner HP, Jan LY, Ruppersberg JP. EMBO J. 1996;15:4093–4099. [PMC free article] [PubMed] [Google Scholar]
- 24.McNicholas CM, Wang W, Ho K, Hebert SC, Giebisch G. Proc Natl Acad Sci USA. 1994;91:8077–8081. doi: 10.1073/pnas.91.17.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ali S, Chen X, Lu M, Xu J-C, Lerea KM, Hebert SC, Wang W. Proc Natl Acad Sci USA. 1998;95:10274–10278. doi: 10.1073/pnas.95.17.10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng WZ, Li XJ, Hilgemann DW, Huang CL. J Biol Chem. 2003;278:16852–16856. doi: 10.1074/jbc.M300619200. [DOI] [PubMed] [Google Scholar]
- 27.Sterling H, Lin DH, Gu RM, Dong K, Hebert SC, Wang WH. J Biol Chem. 2002;277:4317–4323. doi: 10.1074/jbc.M109739200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giebisch G, Wang WH. Kidney Int. 1996;49:1624–1631. doi: 10.1038/ki.1996.236. [DOI] [PubMed] [Google Scholar]
- 29.Strange K. Am J Physiol. 1990;258:F732–F740. doi: 10.1152/ajprenal.1990.258.3.F732. [DOI] [PubMed] [Google Scholar]
- 30.Sansom SC, Stockand JD. Clin Exp Pharmacol Physiol. 1996;23:76–82. doi: 10.1111/j.1440-1681.1996.tb03066.x. [DOI] [PubMed] [Google Scholar]
- 31.Bailey MA, Cantone A, Yan QS, MacGregor GG, Leng Q, Amorim JB, Wang T, Hebert SC, Giebisch G, Malnic G. Kidney Int. 2006;70:51–59. doi: 10.1038/sj.ki.5000388. [DOI] [PubMed] [Google Scholar]
- 32.Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM. Am J Physiol. 2003;285:F998–F1012. doi: 10.1152/ajprenal.00067.2003. [DOI] [PubMed] [Google Scholar]
- 33.Grunnet M, Hay-Scmidt A, Klaerke DA. Biochem Biophys Acta. 2005;1714:114–124. doi: 10.1016/j.bbamem.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 34.Najjar F, Zhou H, Morimoto T, Bruns JB, Li HS, Liu W, Kleyman TR, Satlin LM. Am J Physiol. 2005;289:F922–F932. doi: 10.1152/ajprenal.00057.2005. [DOI] [PubMed] [Google Scholar]
- 35.Gray DA, Frindt G, Palmer LG. Am J Physiol. 2005;289:F117–F126. doi: 10.1152/ajprenal.00471.2004. [DOI] [PubMed] [Google Scholar]
- 36.Robinson MJ, Cobb MH. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 37.Aimond F, Rauzier JM, Bony C, Vassort G. J Biol Chem. 2000;275:39110–39116. doi: 10.1074/jbc.M008192200. [DOI] [PubMed] [Google Scholar]
- 38.Ye CP, Yano S, Tfelt-Hansen J, MacLeod RJ, Ren X, Terwilliger E, Brown EM, Chattopadhyay N. J Neurosci Res. 2004;75:491–498. doi: 10.1002/jnr.10875. [DOI] [PubMed] [Google Scholar]
- 39.Yuan LL, Adams JP, Swank M, Sweatt JD, Johnston D. J Neurosci. 2002:4860–4868. doi: 10.1523/JNEUROSCI.22-12-04860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi H, Asher C, Chigaev A, Yung Y, Reuveny E, Seger R, Garty H. J Biol Chem. 2002;277:13539–13547. doi: 10.1074/jbc.M111717200. [DOI] [PubMed] [Google Scholar]
- 41.Babilonia E, Wei Y, Sterling H, Kaminski P, Wolin MS, Wang WH. J Biol Chem. 2005;280:10790–10796. doi: 10.1074/jbc.M414610200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim HW, New L, Han J, Molkentin JD. J Biol Chem. 2001;276:15913–15919. doi: 10.1074/jbc.M100452200. [DOI] [PubMed] [Google Scholar]
- 43.Zhou X, Wingo CS. Am J Physiol. 1992;263:F1134–F1141. doi: 10.1152/ajprenal.1992.263.6.F1134. [DOI] [PubMed] [Google Scholar]
- 44.Zhou X, Wingo CS. Am J Physiol. 1994;267:F114–F120. doi: 10.1152/ajprenal.1994.267.1.F114. [DOI] [PubMed] [Google Scholar]
- 45.Sun TJ, Zeng WZ, Huang CL. Am J Physiol. 2005;288:F284–F289. doi: 10.1152/ajprenal.00320.2004. [DOI] [PubMed] [Google Scholar]
- 46.Satlin LM, Palmer LG. Am J Physiol. 1997;272:F397–F404. doi: 10.1152/ajprenal.1997.272.3.F397. [DOI] [PubMed] [Google Scholar]