Abstract
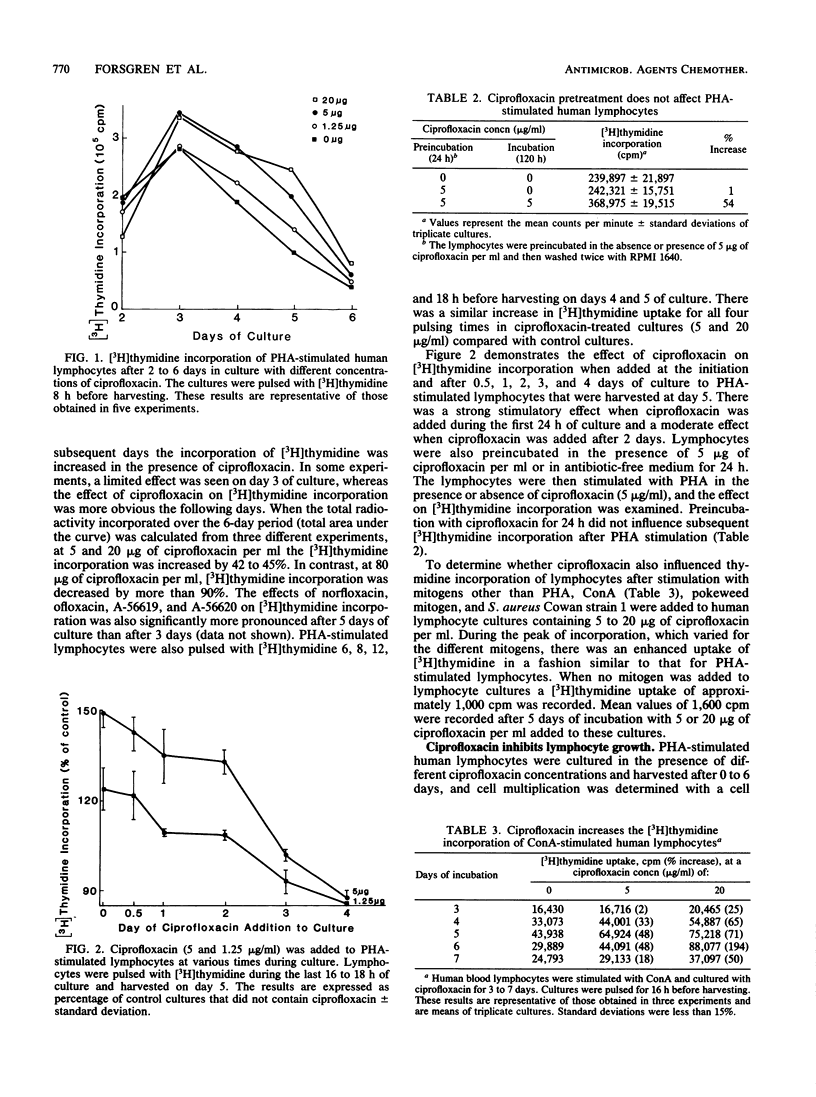
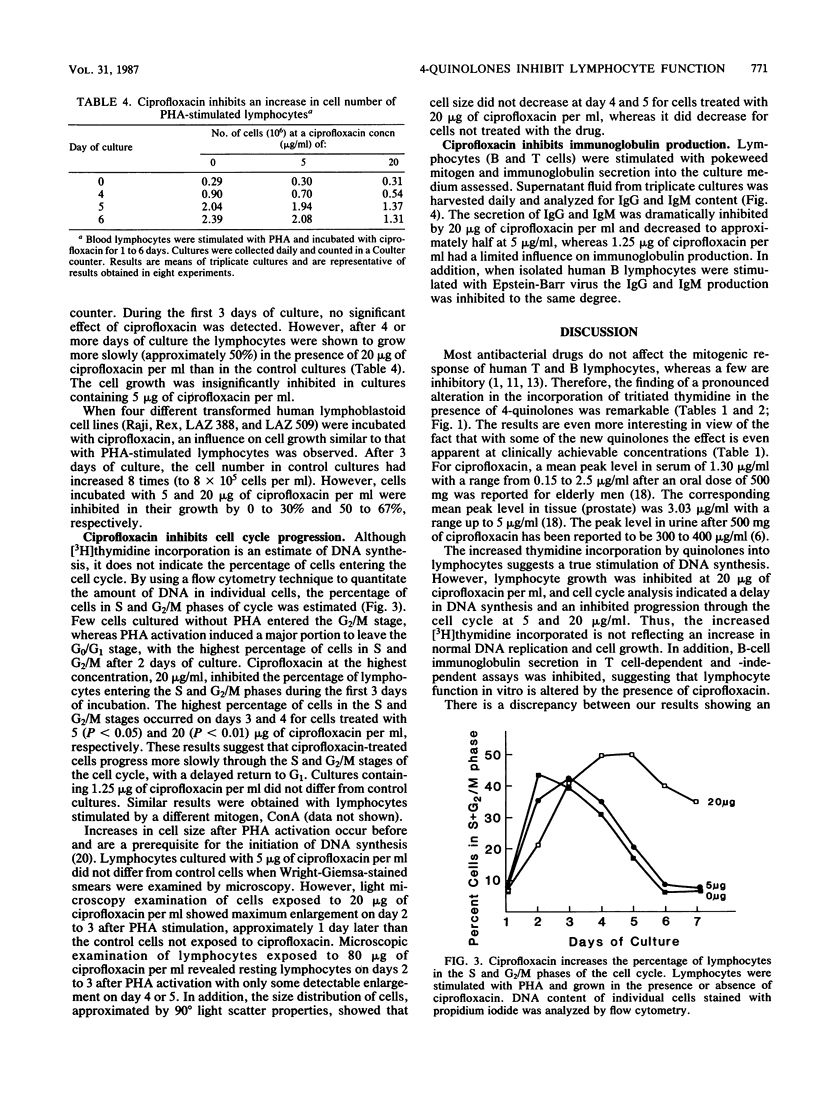
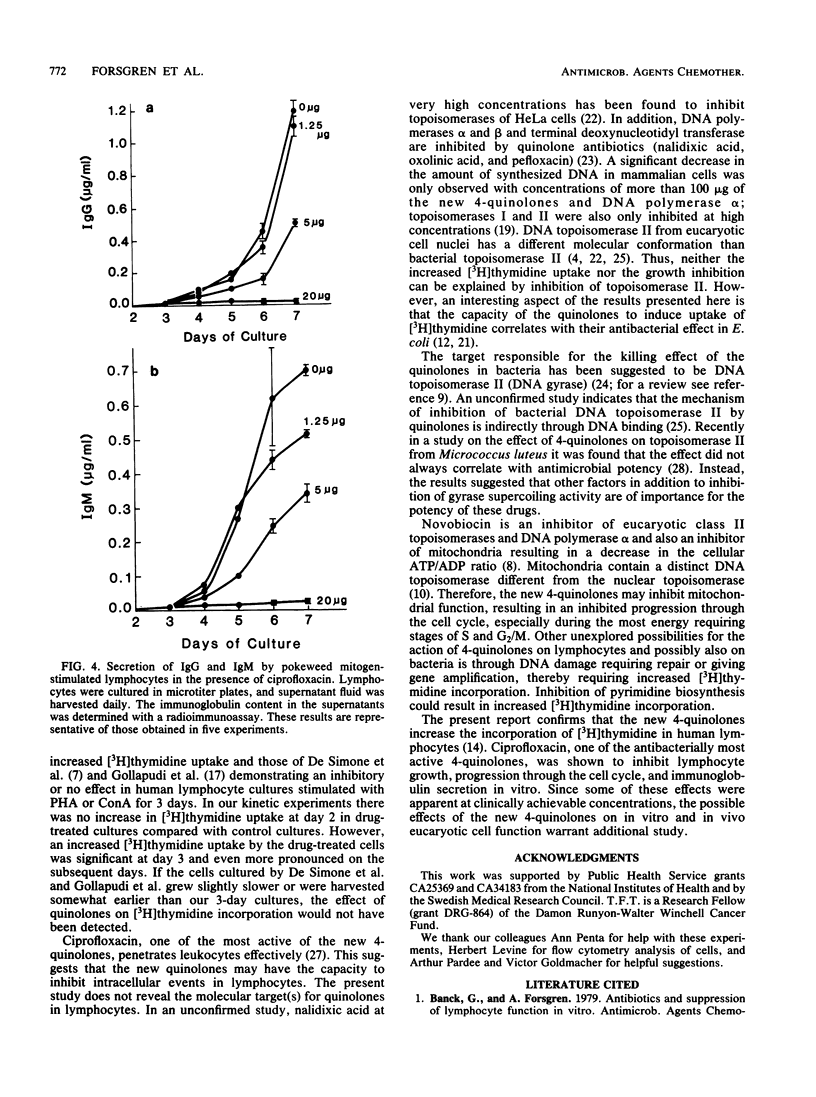
Most antibacterial agents do not affect human lymphocyte function, but a few are inhibitory. In contrast, a pronounced increase in the incorporation of [3H]thymidine in the presence of 4-quinolones was observed in these studies. The uptake of [3H]thymidine into DNA (trichloroacetic acid precipitable) was significantly increased in phytohemagglutinin-stimulated human lymphocytes when they were exposed to eight new 4-quinolone derivatives, ciprofloxacin, norfloxacin, ofloxacin, A-56619, A-56620, amifloxacin, enoxacin, and pefloxacin, at 1.6 to 6.25 micrograms/ml for 5 days. Four less antibacterially active 4-quinolones (nalidixic acid, cinoxacin, flumequine, and pipemidic acid) stimulated [3H]thymidine incorporation only at higher concentrations or not at all. Kinetic studies showed that incorporation of [3H]thymidine was not affected or slightly inhibited by ciprofloxacin 2 days after phytohemagglutinin stimulation but was increased on days 3 to 6. The total incorporation of [3H]thymidine from day 1 to day 6 after phytohemagglutinin stimulation was increased by 42 to 45% at 5 to 20 micrograms of ciprofloxacin per ml. Increased [3H]thymidine incorporation was also seen when human lymphocytes were stimulated with mitogens other than phytohemagglutinin. Ciprofloxacin added at the start of the culture had a more pronounced effect on [3H]thymidine incorporation than when added later. In spite of the apparent increase in DNA synthesis, lymphocyte growth was inhibited by 20 micrograms of ciprofloxacin per ml, and cell cycle analysis showed that ciprofloxacin inhibited progression through the cell cycle. In addition, immunoglobulin secretion by human lymphocytes stimulated by pokeweed mitogen for Epstein-Barr virus was inhibited by approximately 50% at 5 micrograms of ciprofloxacin per ml. These results suggest that the 4-quinolone drugs may also affect eucaryotic cell function in vitro, but additional studies are needed to establish an in vivo relevance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensussan A., Meuer S. C., Schlossman S. F., Reinherz E. L. Delineation of an immunoregulatory amplifier population recognizing autologous Ia molecules. Analysis with human T cell clones. J Exp Med. 1984 Feb 1;159(2):559–576. doi: 10.1084/jem.159.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. L., Yang L., Rowe T. C., Halligan B. D., Tewey K. M., Liu L. F. Nonintercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984 Nov 10;259(21):13560–13566. [PubMed] [Google Scholar]
- Crissman H. A., Steinkamp J. A. Rapid, simultaneous measurement of DNA, protein, and cell volume in single cells from large mammalian cell populations. J Cell Biol. 1973 Dec;59(3):766–771. doi: 10.1083/jcb.59.3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. L., Koup J. R., Williams-Warren J., Weber A., Smith A. L. Pharmacokinetics of three oral formulations of ciprofloxacin. Antimicrob Agents Chemother. 1985 Jul;28(1):74–77. doi: 10.1128/aac.28.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simone C., Baldinelli L., Ferrazzi M., De Santis S., Pugnaloni L., Sorice F. Influence of ofloxacin, norfloxacin, nalidixic acid, pyromidic acid and pipemidic acid on human gamma-interferon production and blastogenesis. J Antimicrob Chemother. 1986 Jun;17(6):811–814. doi: 10.1093/jac/17.6.811. [DOI] [PubMed] [Google Scholar]
- Downes C. S., Ord M. J., Mullinger A. M., Collins A. R., Johnson R. T. Novobiocin inhibition of DNA excision repair may occur through effects on mitochondrial structure and ATP metabolism, not on repair topoisomerases. Carcinogenesis. 1985 Sep;6(9):1343–1352. doi: 10.1093/carcin/6.9.1343. [DOI] [PubMed] [Google Scholar]
- Drlica K. Biology of bacterial deoxyribonucleic acid topoisomerases. Microbiol Rev. 1984 Dec;48(4):273–289. doi: 10.1128/mr.48.4.273-289.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairfield F. R., Bauer W. R., Simpson M. V. Mitochondria contain a distinct DNA topoisomerase. J Biol Chem. 1979 Oct 10;254(19):9352–9354. [PubMed] [Google Scholar]
- Forsgren A., Bergh A. K., Brandt M., Hansson G. Quinolones affect thymidine incorporation into the DNA of human lymphocytes. Antimicrob Agents Chemother. 1986 Mar;29(3):506–508. doi: 10.1128/aac.29.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren A., Bergkvist P. I. Effect of ciprofloxacin on phagocytosis. Eur J Clin Microbiol. 1985 Dec;4(6):575–578. doi: 10.1007/BF02013398. [DOI] [PubMed] [Google Scholar]
- Forsgren A. Comparative in vitro activity of three new quinolone antibiotics against recent clinical isolates. Scand J Infect Dis. 1985;17(1):91–94. doi: 10.3109/00365548509070426. [DOI] [PubMed] [Google Scholar]
- Forsgren A., Svedjelund A., Wigzell H. Lymphocyte stimulation by protein A of Staphylococcus aureus. Eur J Immunol. 1976 Mar;6(3):207–213. doi: 10.1002/eji.1830060312. [DOI] [PubMed] [Google Scholar]
- Gollapudi S. V., Prabhala R. H., Thadepalli H. Effect of ciprofloxacin on mitogen-stimulated lymphocyte proliferation. Antimicrob Agents Chemother. 1986 Feb;29(2):337–338. doi: 10.1128/aac.29.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabe M., Forsgren A., Björk T. Concentrations of ciprofloxacin in serum and prostatic tissue in patients undergoing transurethral resection. Eur J Clin Microbiol. 1986 Apr;5(2):211–212. doi: 10.1007/BF02013991. [DOI] [PubMed] [Google Scholar]
- Hussy P., Maass G., Tümmler B., Grosse F., Schomburg U. Effect of 4-quinolones and novobiocin on calf thymus DNA polymerase alpha primase complex, topoisomerases I and II, and growth of mammalian lymphoblasts. Antimicrob Agents Chemother. 1986 Jun;29(6):1073–1078. doi: 10.1128/aac.29.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrl J. H., Muraguchi A., Fauci A. S. Human B cell activation and cell cycle progression: stimulation with anti-mu and Staphylococcus aureus Cowan strain I. Eur J Immunol. 1984 Feb;14(2):115–121. doi: 10.1002/eji.1830140203. [DOI] [PubMed] [Google Scholar]
- King A., Shannon K., Phillips I. The in-vitro activity of ciprofloxacin compared with that of norfloxacin and nalidixic acid. J Antimicrob Chemother. 1984 Apr;13(4):325–331. doi: 10.1093/jac/13.4.325. [DOI] [PubMed] [Google Scholar]
- Rusquet R., Bonhommet M., David J. C. Quinolone antibiotics inhibit eucaryotic DNA polymerase alpha and beta, terminal deoxynucleotidyl transferase but not DNA ligase. Biochem Biophys Res Commun. 1984 Jun 29;121(3):762–769. doi: 10.1016/0006-291x(84)90744-7. [DOI] [PubMed] [Google Scholar]
- Shen L. L., Pernet A. G. Mechanism of inhibition of DNA gyrase by analogues of nalidixic acid: the target of the drugs is DNA. Proc Natl Acad Sci U S A. 1985 Jan;82(2):307–311. doi: 10.1073/pnas.82.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedder T. F., Boyd A. W., Freedman A. S., Nadler L. M., Schlossman S. F. The B cell surface molecule B1 is functionally linked with B cell activation and differentiation. J Immunol. 1985 Aug;135(2):973–979. [PubMed] [Google Scholar]
- Traub W. H. Intraphagocytic bactericidal activity of bacterial DNA gyrase inhibitors against Serratia marcescens. Chemotherapy. 1984;30(6):379–386. doi: 10.1159/000238297. [DOI] [PubMed] [Google Scholar]
- Zweerink M. M., Edison A. Inhibition of Micrococcus luteus DNA gyrase by norfloxacin and 10 other quinolone carboxylic acids. Antimicrob Agents Chemother. 1986 Apr;29(4):598–601. doi: 10.1128/aac.29.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]



