Abstract
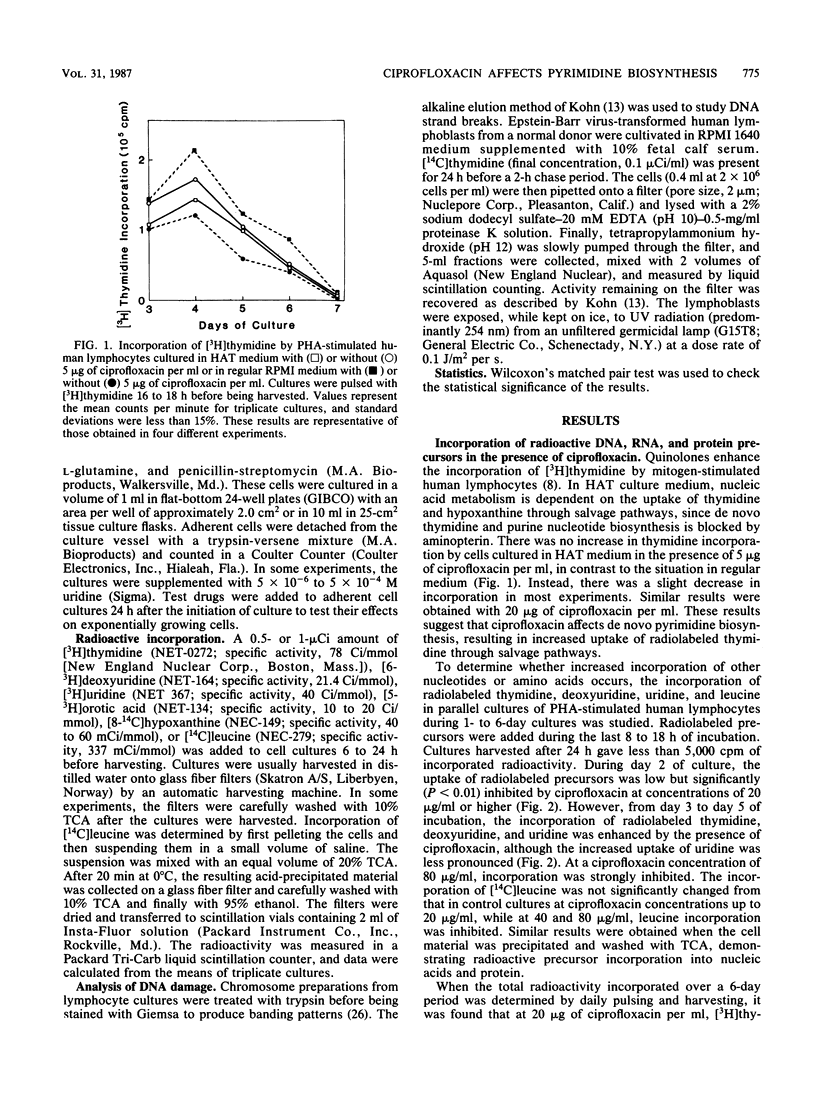
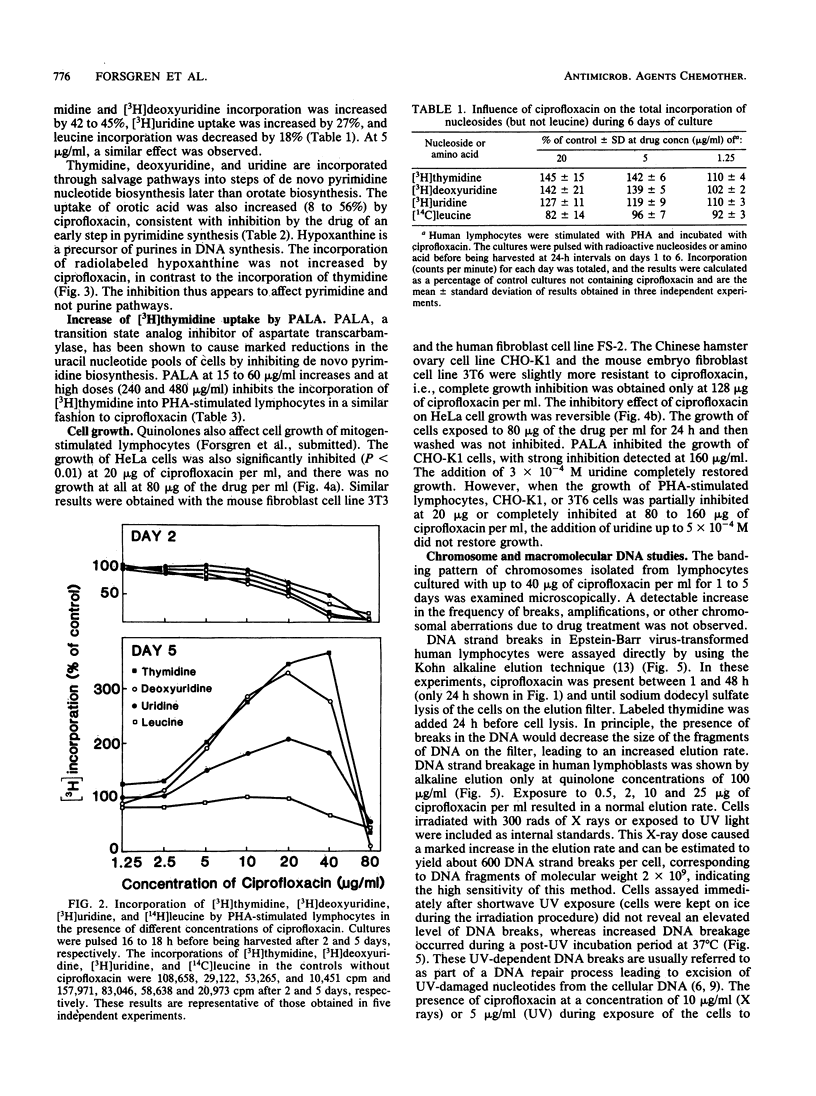
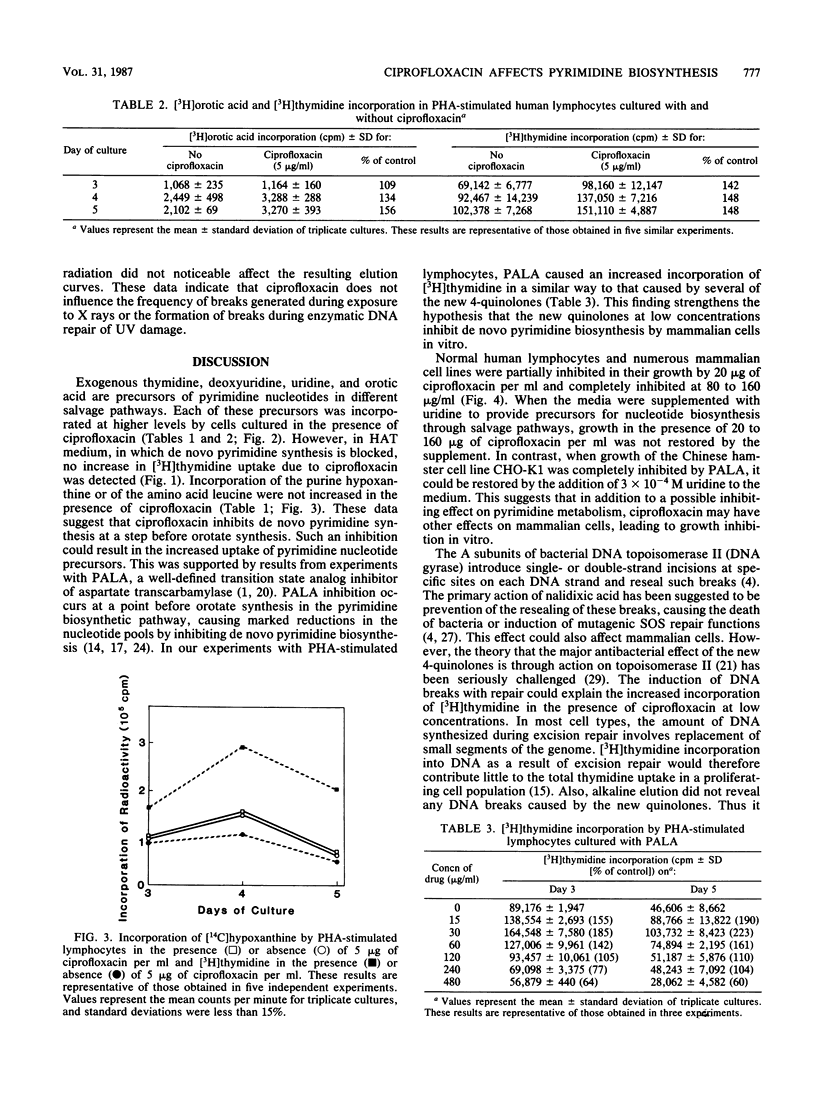
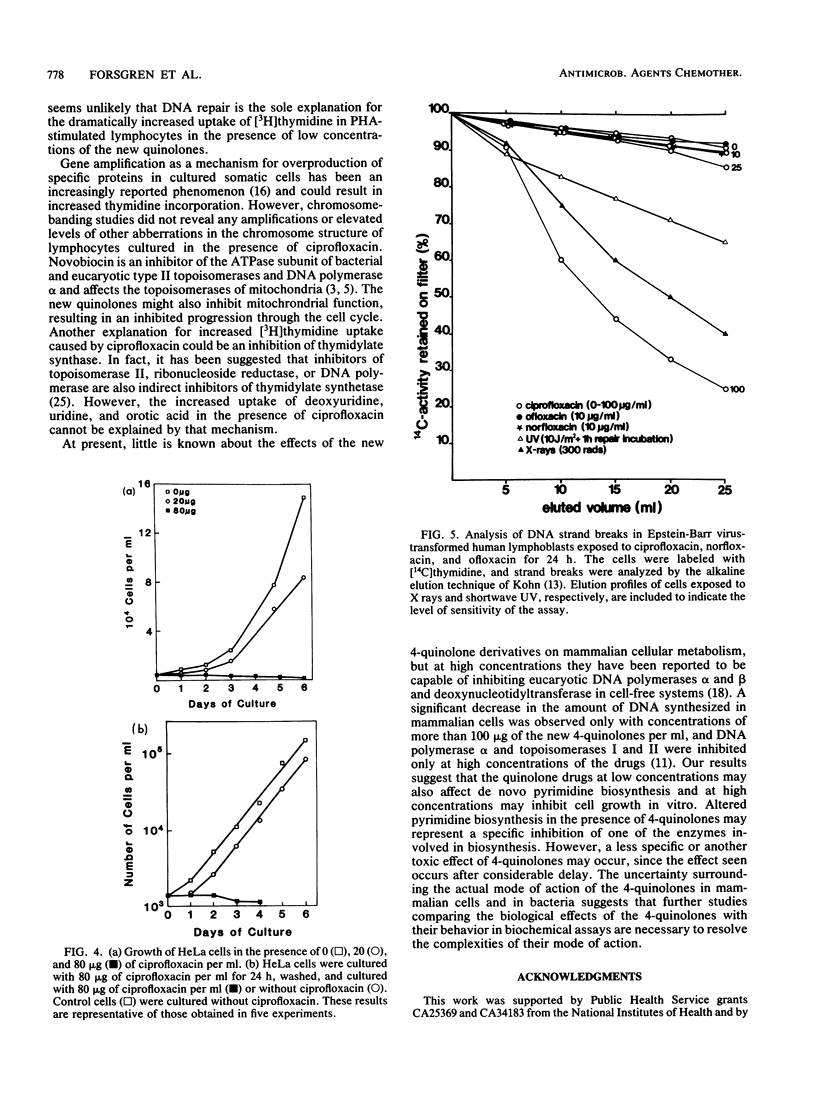
Several of the new 4-quinolones significantly increase the incorporation of [3H]thymidine into the DNA of mitogen-stimulated human lymphocytes. This study suggests that ciprofloxacin inhibits de novo pyrimidine biosynthesis, thereby resulting in a compensatory increase in the uptake of pyrimidine precursors through salvage pathways, and that additional effects may affect eucaryotic cell growth. Incorporation of deoxyuridine, uridine, and orotic acid as well as thymidine was increased in the presence of ciprofloxacin, one of the antibacterially most active of the new 4-quinolones. In contrast, the uptake was decreased in very high concentrations of the drug. Culture in HAT (hypoxanthine, aminopterine, thymidine) medium, which blocks de novo thymidylate synthesis, abrogated the increase in [3H]thymidine incorporation induced by ciprofloxacin. Ciprofloxacin also failed to increase the uptake of [14C]hypoxanthine or leucine, indicating a selective effect on pyrimidine and not on purine nucleotide biosynthesis. N-(Phosphonacetyl)-L-aspartate, an inhibitor of pyrimidine nucleotide biosynthesis, also increased [3H]thymidine incorporation in phytohemagglutinin-stimulated lymphocytes in a fashion similar to ciprofloxacin. The growth of several cell lines was partially inhibited by ciprofloxacin at 20 micrograms/ml and completely inhibited at 80 to 160 micrograms/ml. Growth inhibition by ciprofloxacin could not be restored by the addition of uridine to the medium. Chromosome breaks, gene amplification, or other genetic alterations could not be detected in human lymphocytes incubated with up to 25 micrograms of ciprofloxacin per ml.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collins K. D., Stark G. R. Aspartate transcarbamylase. Interaction with the transition state analogue N-(phosphonacetyl)-L-aspartate. J Biol Chem. 1971 Nov;246(21):6599–6605. [PubMed] [Google Scholar]
- Crumplin G. C., Kenwright M., Hirst T. Investigations into the mechanism of action of the antibacterial agent norfloxacin. J Antimicrob Chemother. 1984 May;13 (Suppl B):9–23. doi: 10.1093/jac/13.suppl_b.9. [DOI] [PubMed] [Google Scholar]
- Downes C. S., Ord M. J., Mullinger A. M., Collins A. R., Johnson R. T. Novobiocin inhibition of DNA excision repair may occur through effects on mitochondrial structure and ATP metabolism, not on repair topoisomerases. Carcinogenesis. 1985 Sep;6(9):1343–1352. doi: 10.1093/carcin/6.9.1343. [DOI] [PubMed] [Google Scholar]
- Drlica K. Biology of bacterial deoxyribonucleic acid topoisomerases. Microbiol Rev. 1984 Dec;48(4):273–289. doi: 10.1128/mr.48.4.273-289.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairfield F. R., Bauer W. R., Simpson M. V. Mitochondria contain a distinct DNA topoisomerase. J Biol Chem. 1979 Oct 10;254(19):9352–9354. [PubMed] [Google Scholar]
- Fornace A. J., Jr, Kohn K. W. DNA-protein cross-linking by ultraviolet radiation in normal human and xeroderma pigmentosum fibroblasts. Biochim Biophys Acta. 1976 Jun 2;435(1):95–103. doi: 10.1016/0005-2787(76)90196-9. [DOI] [PubMed] [Google Scholar]
- Forsgren A., Bergh A. K., Brandt M., Hansson G. Quinolones affect thymidine incorporation into the DNA of human lymphocytes. Antimicrob Agents Chemother. 1986 Mar;29(3):506–508. doi: 10.1128/aac.29.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren A. Comparative in vitro activity of three new quinolone antibiotics against recent clinical isolates. Scand J Infect Dis. 1985;17(1):91–94. doi: 10.3109/00365548509070426. [DOI] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Souza K. S., Tung C., McHugh G. L., Swartz M. N. Genetic and biochemical characterization of norfloxacin resistance in Escherichia coli. Antimicrob Agents Chemother. 1986 Apr;29(4):639–644. doi: 10.1128/aac.29.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussy P., Maass G., Tümmler B., Grosse F., Schomburg U. Effect of 4-quinolones and novobiocin on calf thymus DNA polymerase alpha primase complex, topoisomerases I and II, and growth of mammalian lymphoblasts. Antimicrob Agents Chemother. 1986 Jun;29(6):1073–1078. doi: 10.1128/aac.29.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A., Shannon K., Phillips I. The in-vitro activity of ciprofloxacin compared with that of norfloxacin and nalidixic acid. J Antimicrob Chemother. 1984 Apr;13(4):325–331. doi: 10.1093/jac/13.4.325. [DOI] [PubMed] [Google Scholar]
- Kufe D. W., Egan E. M. Enhancement of 5-fluorouracil incorporation into human lymphoblast ribonucleic acid. Biochem Pharmacol. 1981 Jan 15;30(2):129–133. doi: 10.1016/0006-2952(81)90183-0. [DOI] [PubMed] [Google Scholar]
- Lewensohn R., Killander D., Ringborg U., Lambert B. Increase of uv-induced DNA repair synthesis during blast transformation of human lymphocytes. Exp Cell Res. 1979 Oct 1;123(1):107–110. doi: 10.1016/0014-4827(79)90426-9. [DOI] [PubMed] [Google Scholar]
- Mariani B. D., Schimke R. T. Gene amplification in a single cell cycle in Chinese hamster ovary cells. J Biol Chem. 1984 Feb 10;259(3):1901–1910. [PubMed] [Google Scholar]
- Moyer J. D., Handschumacher R. E. Selective inhibition of pyrimidine synthesis and depletion of nucleotide pools by N-(phosphonacetyl)-L-aspartate. Cancer Res. 1979 Aug;39(8):3089–3094. [PubMed] [Google Scholar]
- Rusquet R., Bonhommet M., David J. C. Quinolone antibiotics inhibit eucaryotic DNA polymerase alpha and beta, terminal deoxynucleotidyl transferase but not DNA ligase. Biochem Biophys Res Commun. 1984 Jun 29;121(3):762–769. doi: 10.1016/0006-291x(84)90744-7. [DOI] [PubMed] [Google Scholar]
- Shen L. L., Pernet A. G. Mechanism of inhibition of DNA gyrase by analogues of nalidixic acid: the target of the drugs is DNA. Proc Natl Acad Sci U S A. 1985 Jan;82(2):307–311. doi: 10.1073/pnas.82.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. T. Mutational resistance to 4-quinolone antibacterial agents. Eur J Clin Microbiol. 1984 Aug;3(4):347–350. doi: 10.1007/BF01977492. [DOI] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swyryd E. A., Seaver S. S., Stark G. R. N-(phosphonacetyl)-L-aspartate, a potent transition state analog inhibitor of aspartate transcarbamylase, blocks proliferation of mammalian cells in culture. J Biol Chem. 1974 Nov 10;249(21):6945–6950. [PubMed] [Google Scholar]
- Wang H. C., Fedoroff S. Banding in human chromosomes treated with trypsin. Nat New Biol. 1972 Jan 12;235(54):52–54. doi: 10.1038/newbio235052a0. [DOI] [PubMed] [Google Scholar]
- Witkin E. M., Wermundsen I. E. Targeted and untargeted mutagenesis by various inducers of SOS functions in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):881–886. doi: 10.1101/sqb.1979.043.01.095. [DOI] [PubMed] [Google Scholar]
- Yamagishi J., Furutani Y., Inoue S., Ohue T., Nakamura S., Shimizu M. New nalidixic acid resistance mutations related to deoxyribonucleic acid gyrase activity. J Bacteriol. 1981 Nov;148(2):450–458. doi: 10.1128/jb.148.2.450-458.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweerink M. M., Edison A. Inhibition of Micrococcus luteus DNA gyrase by norfloxacin and 10 other quinolone carboxylic acids. Antimicrob Agents Chemother. 1986 Apr;29(4):598–601. doi: 10.1128/aac.29.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- veer Reddy G. P., Pardee A. B. Inhibitor evidence for allosteric interaction in the replitase multienzyme complex. Nature. 1983 Jul 7;304(5921):86–88. doi: 10.1038/304086a0. [DOI] [PubMed] [Google Scholar]



