Abstract
Context: Control of the trunk segment in landing has been implicated as a contributing factor to the higher incidence of anterior cruciate ligament injuries in females than in males. Investigating the sex-specific abdominal activation strategies during landing lends insight into mechanisms contributing to control of the trunk segment.
Objective: To examine the abdominal activation strategies used by males and females during a landing task.
Design: Mixed-model (between-subjects and within-subjects) design.
Setting: Laboratory.
Patients or Other Participants: Healthy, recreationally active males (n = 20, age = 23 ± 4.8 years, height = 1.8 ± 0.1 m, mass = 79.6 ± 9.9 kg, body mass index = 24.8 ± 2.7 kg/ m 2) and females (n = 22, age = 20.8 ± 4.8 years, height = 1.7 ± 0.1 m, mass = 64.1 ± 9.2 kg, body mass index = 22.9 ± 2.6 kg/m 2).
Intervention(s): Subjects performed 5 double-leg landings from a box height of 60 cm.
Main Outcome Measure(s): Male and female activation amplitudes for the rectus abdominis (RA), external oblique (EO), and transversus abdominis and lower fibers of the internal oblique (TrA-IO) muscles during preactivation (150-millisecond interval just before landing) and after impact (150-millisecond interval immediately after ground contact).
Results: Males had greater TrA-IO activation than females ( P < .05). Males preferentially activated the TrA-IO muscles relative to the RA and EO, whereas females demonstrated no significant muscle differences. Males and females also differed by phase, with males having more TrA-IO activation than females during the preactivation landing phase ( P < .05) but not during the postimpact phase. The TrA-IO was the only muscle to significantly differ by landing phase, decreasing from preactivation to postimpact ( P < .05).
Conclusions: Males used different abdominal muscle activation strategies than females in landing. The efficacy of these muscle activation strategies to control the trunk should be assessed through trunk kinematic and kinetic measures in future studies.
Keywords: sex differences, trunk muscle recruitment
The incidence of anterior cruciate ligament (ACL) injuries is higher in females than males, and the direct economic cost associated with ACL tears exceeds 1 billion dollars annually. 1–4 The sex discrepancy in ACL injuries and the injury's associated economic costs and long-term complications (eg, osteoarthritis 5) have collectively led to research investigating sex differences in lower extremity landing mechanics. 6–8 The “position of no return” describes a collective posture of foot pronation, tibial external rotation, femoral internal rotation, and an awkward or excessively anteriorly flexed trunk position that theoretically result in an ACL injury. 9 Although authors have examined lower extremity biomechanical and neuromuscular strategies inherent to males and females in landing, 6, 10, 11 research on factors contributing to trunk control in landing has been limited. The head, arms, and trunk segment comprise more than 60% of the body's mass, 12 and the position and orientation of all segments in the kinetic chain influence the ground reaction force and, thus, the forces and moments placed on the lower extremity joints. 13 Thus, investigations to determine the sex-specific neuromuscular strategies to control the trunk during landing seem warranted, as these may ultimately help to clarify factors contributing to trunk control and the position of no return.
Muscles contributing to trunk control during dynamic motion can be separated into local and global muscles depending on their anatomical orientation and function. 14 The local abdominal muscles, the transversus abdominis (TrA) and lower fibers of the internal oblique (IO), are key dynamic stabilizing muscles of the spine, lumbopelvic region, and whole trunk-pelvis segment, collectively comprising the “core.” 15–19 Their stabilizing role has been demonstrated during reaction-based tasks involving rapid arm and leg movements, 15, 16 during walking and running, 20, 21 lifting and lowering, 22 and landing. 17 The local abdominal muscles activate to increase intra-abdominal pressure, which enhances lumbar spine and sacroiliac joint stiffness, resulting in true lumbopelvic and trunk control. 18, 19 The local abdominal muscles stabilize the spine and trunk as a whole, whereas the rectus abdominis (RA), external obliques (EO), and erector spinae (ES), also known as the global trunk muscles, counteract any perturbations to the body's center of mass and, thus, primarily control trunk position relative to the body's base of support. 15, 23 Further, the local abdominal and ES muscles are augmented in situations requiring increased trunk support, such as during squatting on an unstable surface. 24 This finding demonstrates the adaptability of the neuromuscular system in stabilizing the trunk when enhanced support is needed. 24 The global and local abdominal muscles have independent but equally important functions to stabilize the trunk, so it would seem that people who cannot adequately stabilize the trunk may also have underlying abdominal muscle activation deficiencies.
The functional importance of the global and local abdominal muscle groups when increased demands are placed on the trunk has been well demonstrated. 25–27 Further, these trunk muscle activation characteristics have been shown to be sex specific: in preparing for a sudden trunk load, females augment the RA and EO muscles more than males. 25 This result indicates that females may use a trunk stabilization strategy that depends more on the global abdominal muscles than do males. Research is therefore needed to determine if sex-specific abdominal activation strategies occur during landing, a task requiring abdominal recruitment to control the momentum of the trunk segment at impact. Thus, our specific aim was to investigate the sex-specific abdominal muscle activation strategies during the time intervals just before (preactivation) and immediately after (postimpact) landing. Based on previous findings, 17, 18, 20, 25 we hypothesized that although the local abdominals would be the primary abdominal muscle group activated during landing, females would have significantly higher global abdominal activation (RA and EO) than males during landing.
METHODS
Design and Setting
We followed a mixed-model, repeated-measures design in which males and females were compared with regard to each of the abdominal muscles across preactivation and postimpact landing intervals. All testing procedures were approved by the University Institutional Review Board for the Protection of Human Subjects. Testing was performed in a laboratory setting. Dependent variables were muscle amplitudes for the RA, EO, and TrA-IO during preactivation and postimpact. The independent variables were sex (male, female) and landing phase (preactivation, postimpact).
Subjects
Forty-two subjects, 20 males (age = 23 ± 4.8 years, height = 1.8 ± 0.1 m, mass = 79.6 ± 9.9 kg, body mass index = 24.8 ± 2.7 kg/m 2) and 22 females (age = 20.8 ± 4.8 years, height = 1.7 ± 0.1 m, mass = 64.1 ± 9.2 kg, body mass index = 22.9 ± 2.6 kg/m 2), participated in the study. Subjects qualified as being recreationally active if they engaged in physical activity for a minimum of 30 minutes at least 3 times a week. In addition, all subjects had prior recreational experience in jumping and landing activities, including basketball, volleyball, and gymnastics. All subjects were apparently healthy individuals who reported no current injuries or history of surgery to the lower extremity or low back. Additional exclusionary criteria included a past history of receiving any treatment for low back pain. Screening for previous low back conditions (through verbal communication) was essential, as disrupted and compensatory abdominal activation patterns are associated with low back injuries. 28, 29
Instrumentation
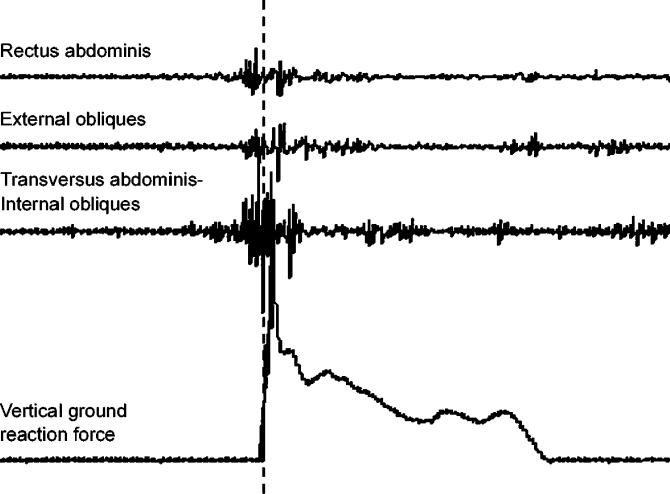
Surface electromyography (EMG) of the abdominal muscles was acquired using a Myopac 2000 system (Run Technologies, Mission Viejo, CA), and the subsequent signal was stored, processed, and analyzed with DATAPAC 2k2 lab application software (Run Technologies). All surface EMG data were sampled at 1000 Hz. This EMG system has a frequency bandwidth of 10 to 1000 Hz, common mode rejection ratio of 90 dB, and an internal sampling rate of 8 KHz. Two forceplates (model 4060-nonconducting; Bertec Corp, Columbus, OH) acquired ground reaction forces for both limbs. A separate computer sampled the forceplate data at 1000 Hz (Motion Monitor Software; Innovative Sports Training, Chicago, IL). The vertical ground reaction signal was then interfaced with the DATAPAC software to trigger data acquisition and to indicate the time of ground contact ( Figure 1). A ground reaction force of 40 N triggered data acquisition for each landing trial, and surface EMG data were recorded for 500 milliseconds before and 1000 milliseconds after initial contact with the forceplate.
Figure 1. Representative abdominal electromyographic data during landing. Dotted vertical line indicates initial ground contact.
Procedures
Upon arrival at the laboratory for data collection, all subjects first gave written informed consent. The primary investigator then demonstrated the double-leg landing from a 60-cm-high box, and subjects practiced until they were comfortable with the task. Instructions to every subject included the following: hold the hands at the sides of hips with the thumbs on top of the hips and fingers pointing downward at all times; start with both feet at the edge of the box; reach straight out with the preferred leg and shift the weight of the hips forward off the box; and land on both feet at the same time and return to a normal standing position all in one smooth and natural manner. The subject's preferred leg was determined by observing which foot was most frequently used in practicing the task. Subjects were specifically instructed not to jump up or out from the box or lower the body down. After the subjects were comfortable performing the task, preparation for surface EMG followed. We chose a drop landing task instead of a drop jumping task based on the goals of our study. Although both tasks require a change in vertical momentum to decelerate the body's center of mass, the drop jumping task is usually goal oriented in that subjects jump for maximal height, whereas the goal of the drop landing task is to land in a smooth and coordinated manner. We believed that the drop jumping task would hinder the interpretation of our results because jumping for maximal height (ie, the effort put forth by the subjects) might vary and further confound our sex comparisons.
Surface EMG preparation consisted of scrubbing the skin with an alcohol pad to enhance surface contact with the electrode, followed by placement of pairs of bipolar Ag-AgCl electrodes (Blue Sensor NF-00-S; Ambu Products, Friedberg, Germany) at 3 abdominal sites. Electrode placement for the TrA-IO muscles was 2 cm medial and inferior to the anterior superior iliac spine. 30, 31 This location has been used to assess activation profiles of the TrA-IO and has been viewed as the best surface location for evaluating TrA function. 30, 31 Electrode placement for the RA was 2 cm lateral to the umbilicus, whereas EO electrode placement was 12 cm lateral to the umbilicus at an oblique angle of 45° to coincide with the muscle's fiber orientation. 30 All electrodes had an interelectrode distance of 1 cm. A reference electrode was placed on the right tibia. All electrode wires and electrodes were secured to the abdomen with athletic tape to minimize wire and electrode movement artifact upon impact. Submaximal voluntary isometric contractions (SMVICs) of all 3 abdominal muscles were used to normalize the EMG data. Subjects were positioned supine with the hips flexed to 45° and feet flat on the floor. They were required to lift the feet off the floor approximately 2.5 cm and hold for 3 seconds. Three trials were performed, and the average of these trials was used to normalize the landing EMG. Visual inspection during all SMVICs confirmed a consistent signal from each muscle. This procedure was chosen to normalize the EMG data because it provides excellent reliability for activation of all abdominal muscles in both healthy and injured populations. 32 In addition, we evaluated the internal consistency of the abdominal surface EMG on a trial-by-trial basis. Intraclass correlation coefficients (ICC[2,1]) computed on each of the abdominal variables were good ( r = .82 to .92), thereby justifying our data collection methods.
Following the SMVIC procedure, all subjects were then instructed to drop from a 60-cm-high box and land “as naturally” as they could, with the feet landing on separate, side-by-side forceplates. Subjects performed 5 double-leg drop landing trials.
Data Processing and Reduction
All surface EMG signals (SMVIC and trial data) were band-pass filtered between 10 and 350 Hz using a fourth-order, zero-lag digital Butterworth filter and were then full-wave rectified. For the landing trials, the signals were then integrated for 2 time windows of interest: (1) preactivity, defined as the 150-millisecond time interval just before ground contact, and (2) postimpact activity, defined as the 150-millisecond time interval immediately after initial contact with the forceplate. To account for heartbeat artifact, integrated abdominal signals acquired with the subject in a quiet, supine position for 150 milliseconds were subtracted from the integrated surface EMG trial data (using the same 150-millisecond time intervals) for each muscle. After initial band-pass filtering and rectification, the middle 150 milliseconds of each of the 3 SMVIC trials were used to compute a mean SMVIC with the same time constant as the landing data (preactivity and postimpact). All EMG data were then imported into a spreadsheet program and normalized to the SMVIC.
Statistical Analyses
Means of the 5 landing trials were entered for statistical analysis. We computed a 3 (muscle) × 2 (phase of landing) × 2 (sex) mixed-model analysis of variance to compare muscle activation (RA, EO, TrA-IO) and phase of landing (preactivity and postimpact) between the sexes. To further analyze significant interactions, we calculated post hoc Tukey Honestly Significant Difference tests. Alpha levels were set a priori at .05 for all analyses.
RESULTS
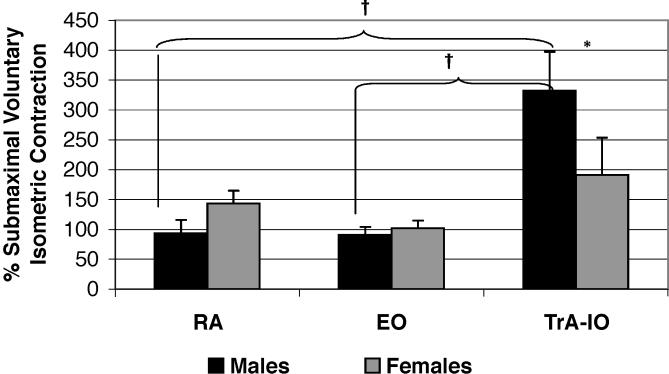
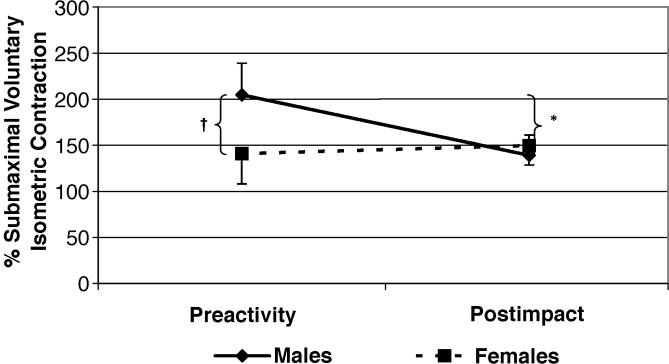
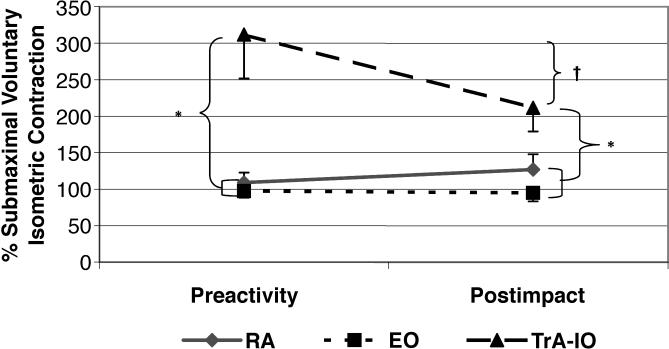
The Table displays the means and standard deviations for all dependent variables used in the mixed-model analysis of variance. A significant sex-by-muscle interaction (F 2,80 = 3.76, P < .05, η 2 = .086, 1 − β = .67; Figure 2) demonstrated that males produced higher TrA-IO than RA and EO amplitudes, whereas females showed no differences among muscles. In addition, females had significantly lower TrA-IO activation amplitudes than males. Males and females also differed by phase (F 1,40 = 5.53, P < .05, η 2 = .121, 1 − β = .63; Figure 3), with males producing significantly higher preactivation amplitudes than females. The males then showed decreased muscle activation amplitudes from preactivation to postimpact, resulting in values that were not significantly different than those of the females. Females showed no change in muscle activation from preactivation to postimpact. Finally, a muscle-by-phase interaction (F 2,80 = 9.90, P < .05, η 2 = .198, 1 − β = .98; Figure 4) demonstrated that although activation of the TrA-IO was greater than that of the RA and EO during both preactivation and postimpact, a significant decrease in TrA-IO from preactivation to postimpact was evident, whereas no change was noted across phase of landing for the RA or EO. No significant sex-by-muscle-by-phase interaction (F 2,80 = .62, P = .54, η 2 = .015, 1 − β = .15) and no main effect for sex (F 1,40 = .52, P = .48, η 2 = .013, 1 − β = .11) were seen.
Muscle Amplitudes (Percentage of Submaximal Voluntary Isometric Contractions) Between the Landing Phases and the Sexes (Mean ± SD).
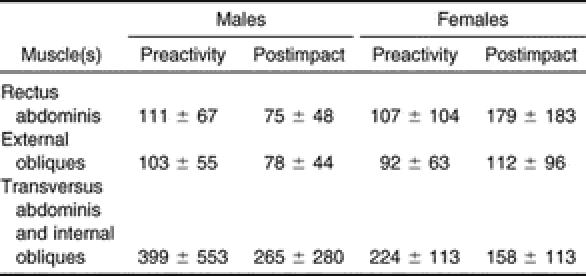
Figure 2. Abdominal muscle activation between the sexes. RA indicates rectus abdominis; EO, external obliques; TrA-IO, transversus abdominis–internal obliques; *, TrA-IO activation in males was significantly greater than in females ( P < .05); and †, in males, the TrA-IO activation was significantly greater than activation of the RA and EO ( P < .05) .
Figure 3. Abdominal activation by phase of landing between the sexes. SMVIC indicates submaximal voluntary isometric contraction; *, significantly decreased abdominal activation in males from preactivity to postimpact ( P < .05); and †, significantly greater preactivation amplitudes in males than in females ( P < .05) .
Figure 4. Abdominal muscle activation across phase of landing. SMVIC indicates submaximal voluntary isometric contraction; RA, rectus abdominis; EO, external obliques; TrA-IO, transversus abdominis–internal obliques; *, TrA-IO preactivation and postimpact amplitudes were significantly greater than RA and EO preactivation and postimpact amplitudes ( P < .05); and †, TrA-IO amplitude significantly decreased across phase of landing from preactivation to postimpact ( P < .05) .
DISCUSSION
Our primary findings were that males recruited higher TrA-IO amplitudes than females, but no sex differences were demonstrated in activation of the RA and EO muscles. This sex difference in TrA-IO amplitude was largely due to greater activation of the TrA-IO in males relative to females in preparation for landing but not postimpact. These findings collectively reveal that males preferentially activated the local abdominals (TrA-IO) over the RA and EO muscles in preparation for landing, whereas females showed no preferential abdominal muscle recruitment.
In the landing task, no differences were noted in global muscle activation (RA or EO) across phase of landing or between the sexes. This is contrary to the findings of Granata et al, 25 who showed that females recruited significantly higher RA and EO amplitudes than males in preparation for a sudden trunk load (ie, a sudden weight was applied to the hands, mimicking lifting conditions). During postimpact, we did see a trend toward higher RA recruitment in females than in males, although these differences were not statistically significant, most likely because of the large SDs present in the females ( P > .05, Cohen D effect size = .90; Table). Based on the statistical findings of our study, we cannot support our initial hypothesis that females would recruit higher RA and EO amplitudes than males.
The larger TrA-IO amplitudes relative to the RA and EO support the role of the local abdominals as dynamic trunk stabilizers and partially confirm our hypothesis. However, this finding was only evident in the male subjects. The local abdominal muscles are the primary abdominal muscles responsible for modulating intra-abdominal pressure (IAP). 17, 22 As a result, we expected large TrA-IO preactivation amplitudes (coinciding with IAP development) in preparation for impact with the ground. Although we did not directly measure IAP, Cresswell and Thorstensson 18 showed that IAP develops before ground contact in preparation for landing. The functional importance of local abdominal activation and IAP development in controlling the trunk has been demonstrated during various reaction-based tasks as well as during walking and running. 20, 21, 33 Our findings also support the importance of local abdominal activation as a dynamic stabilizer of the spine, lumbopelvic, and trunk segments in landing. Our initial hypothesis that the local abdominals would be the primary abdominal muscles recruited relative to the RA and EO is, therefore, rejected, because this was only evident in the male subjects during the preactivation phase of landing.
The activation strategies we observed in the female subjects indicate that they employed an abdominal recruitment strategy that similarly activated the global and local muscles. This activation strategy may indicate a reduced ability to stabilize the trunk in landing. Previous authors showed that activation of the local abdominals to achieve adequate levels of IAP and to stabilize the lumbar spine and trunk are essential in tasks such as walking and running 20, 21 and in landing. 17 Without appropriate activation of the local abdominals, the global abdominal muscles must compensate in order to provide adequate control of trunk motion. 29 As these compensatory activation strategies occur, the heightened global activation strategy may compromise the person's ability to stabilize the trunk, especially if fatigue becomes a factor, such as over the course of repeated trunk loading episodes. 25, 27 A trunk loading stress occurs every time a person lands from a jump; thus, the female athlete may not be able to adequately meet these demands over the course of a game as a result of the activation strategies employed. In addition, as the global abdominals are instrumental in controlling the trunk segment in the presence of an external load 34 and/or during a reaction-based situation, 15, 16 their ability to react and stabilize the trunk may be compromised if their activation levels are already elevated as a result of inadequate local abdominal activation.
The final 2 discussion points are centered on alternative muscle activation strategies in landing and represent possible explanations for the sex discrepancies noted in our study. Although we are only speculating at this time because we did not acquire data from other muscles, these theoretic explanations are based on the current evidence from the literature. The sex discrepancy in local abdominal activation may be explained by the idea that females use alternative muscle activation strategies by other trunk and pelvic muscles to modulate the IAP and control the trunk. Our findings are limited to the local and global abdominal muscles, but the pelvic floor and diaphragm are also instrumental in modulating the IAP, along with the local abdominals; thus, females may have selectively recruited the pelvic floor muscles to develop and maintain IAP during landing. 35 Although the local abdominal muscles contribute to the development of IAP and, in turn, the trunk extensor moment, 18, 36, 37 females may also have preferentially activated the ES muscles to control the forward momentum of the trunk during landing. Given the kinesiologic function of the ES in extending the trunk, this factor may also help to explain why males experienced a significant decrease in local abdominal activation across phase of landing. It is quite possible that the local abdominal muscles preactivate to develop a functional level of IAP before landing, 17 whereas after impact, the ES primarily controls forward trunk flexion. To assess the efficacy of different muscle activation strategies in controlling the trunk during landing, future researchers should investigate how sex-specific abdominal and back muscle activation patterns relate to trunk and pelvis kinematic and kinetic function.
Limitations
The use of surface EMG to represent local abdominal activation includes contributions of the lower fibers of the IO, and, therefore, results cannot be attributed to the contributions of the TrA alone. At 2 cm medial and inferior to the anterior superior iliac spine, the TrA and IO are horizontally oriented and superficial to the skin, 38 and in some cadavers, these muscles are fused at this location. 31, 38 As a result, these muscles cannot be completely isolated from one another. The use of surface EMG to represent deep abdominal activation profiles may also be considered a limitation of our study. However, McGill et al 30 compared surface EMG with intramuscular EMG and demonstrated that surface electrodes placed 2 cm medial and inferior to the anterior superior iliac spine provided a valid representation of the activation profiles of the deep TrA. Others have also used this location to detect TrA onset timing relative to upper limb movement. 31 Although intramuscular EMG might have provided more appropriate information specific to the TrA and not the IO, we believe the highly dynamic nature of the landing task would have caused errant movement of the needle electrode. In addition, by using surface EMG in the current study, the trial-by-trial reliability of each of the abdominal variables was good ( r = .82 to .92). Therefore, we believe that use of surface EMG to represent TrA-IO activation was justified given our highly dynamic task, our variables of interest, the established reliability of our measures, and our hypothesis.
CONCLUSIONS
Abdominal muscle activation strategies used by males and females during landing demonstrate that males preferentially activated the local abdominal muscles (TrA-IO) in preparation for landing, whereas females showed no significant differences in abdominal activation during preactivation or postimpact. The dominant local abdominal activation strategy used by males in landing is consistent with previous findings highlighting the local abdominal muscles as trunk stabilizers. Although females did not preferentially activate the local abdominal muscles, we cannot determine from the current study whether this reflects an inability to control the trunk, as alternative muscle activation strategies to stabilize the trunk may have been employed. Future researchers will need to investigate both the abdominal and back muscle activation strategies inherent in landing and test the efficacy of these sex-specific trunk muscle activation patterns through trunk kinematic and kinetic measurements. Once an “optimal” abdominal muscle activation pattern to control the trunk in landing is identified, clinicians may then begin using the evidence to guide exercise prescription and, thus, ultimately to minimize the chances of ACL injuries occurring in the female athlete.
Acknowledgments
We thank Dr Richard Luecht for his valuable statistical consultation and Al Cody for his laboratory technical expertise throughout the duration of this study.
REFERENCES
- Agel J, Arendt EA, Bershadsky B. Anterior cruciate ligament injury in National Collegiate Athletic Association basketball and soccer: a 13-year review. Am J Sports Med. 2005;33:524–530. doi: 10.1177/0363546504269937. [DOI] [PubMed] [Google Scholar]
- Arendt E, Agel J, Dick R. Anterior cruciate ligament injury patterns among collegiate men and women. J Athl Train. 1999;34:86–92. [PMC free article] [PubMed] [Google Scholar]
- Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer: NCAA data and review of literature. Am J Sports Med. 1995;23:694–701. doi: 10.1177/036354659502300611. [DOI] [PubMed] [Google Scholar]
- Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. CDC Injury Research Agenda. Atlanta, GA: Centers for Disease Control and Prevention; 2002.
- Nelson F, Billinghurst RC, Pidoux I. Early post-traumatic osteoarthritis-like changes in human articular cartilage following rupture of the anterior cruciate ligament. Osteoarthr Cartilage. 2006;14:114–119. doi: 10.1016/j.joca.2005.08.005. et al. [DOI] [PubMed] [Google Scholar]
- Decker MJ, Torry MR, Wyland DJ, Sterett WI, Steadman JR. Gender differences in lower extremity kinematics, kinetics and energy absorption during landing. Clin Biomech (Bristol, Avon) 2003;18:662–669. doi: 10.1016/s0268-0033(03)00090-1. [DOI] [PubMed] [Google Scholar]
- Ford KR, Myer GD, Smith RL, Vianello RM, Seiwert SL, Hewett TE. A comparison of dynamic coronal plane excursion between matched male and female athletes when performing single leg landings. Clin Biomech (Bristol, Avon) 2006;21:33–40. doi: 10.1016/j.clinbiomech.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Garrison JC, Hart JM, Palmieri RM, Kerrigan DC, Ingersoll CD. Lower extremity EMG in male and female college soccer players during single-leg landing. J Sport Rehabil. 2005;14:48–57. [Google Scholar]
- Ireland ML, Gaudette M, Crook S. ACL injuries in the female athlete. J Sport Rehabil. 1997;6:97–110. [Google Scholar]
- Zhang SN, Bates BT, Dufek JS. Contributions of lower extremity joints to energy dissipation during landings. Med Sci Sports Exerc. 2000;32:812–819. doi: 10.1097/00005768-200004000-00014. [DOI] [PubMed] [Google Scholar]
- Devita P, Skelly WA. Effect of landing stiffness on joint kinetics and energetics in the lower extremity. Med Sci Sports Exerc. 1992;24:108–115. [PubMed] [Google Scholar]
- LeVeau BF. Biomechanics of Human Motion. 3rd ed. Philadelphia, PA: WB Saunders; 1992.
- McNitt-Gray JL, Hester DME, Mathiyakom W, Munkasy BA. Mechanical demand and multijoint control during landing depend on orientation of the body segments relative to the reaction force. J Biomech. 2001;34:1471–1482. doi: 10.1016/s0021-9290(01)00110-5. [DOI] [PubMed] [Google Scholar]
- Bergmark A. Stability of the lumbar spine: a study in mechanical engineering. Acta Orthop Scand Suppl. 1989;230:1–54. doi: 10.3109/17453678909154177. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Richardson CA. Feedforward contraction of transversus abdominis is not influenced by the direction of arm movement. Exp Brain Res. 1997;114:362–370. doi: 10.1007/pl00005644. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Richardson CA. Contraction of the abdominal muscles associated with movement of the lower limb. Phys Ther. 1997;77:132–144. doi: 10.1093/ptj/77.2.132. [DOI] [PubMed] [Google Scholar]
- Cresswell AG, Blake PL, Thorstensson A. The effect of an abdominal muscle training program on intra-abdominal pressure. Scand J Rehabil Med. 1994;26:79–86. [PubMed] [Google Scholar]
- Cresswell AG, Thorstensson A. Changes in intra-abdominal pressure, trunk muscle activation and force during isokinetic lifting and lowering. Eur J Appl Physiol Occup Physiol. 1994;68:315–321. doi: 10.1007/BF00571450. [DOI] [PubMed] [Google Scholar]
- Richardson CA, Snijders CJ, Hides JA, Damen L, Pas MS, Storm J. The relation between the transversus abdominis muscles, sacroiliac joint mechanics, and low back pain. Spine. 2002;27:399–405. doi: 10.1097/00007632-200202150-00015. [DOI] [PubMed] [Google Scholar]
- Saunders SW, Rath D, Hodges PW. Postural and respiratory activation of the trunk muscles changes with mode and speed of locomotion. Gait Posture. 2004;20:280–290. doi: 10.1016/j.gaitpost.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Saunders SW, Schache A, Rath D, Hodges PW. Changes in three dimensional lumbo-pelvic kinematics and trunk muscle activity with speed and mode of locomotion. Clin Biomech (Bristol, Avon) 2005;20:784–793. doi: 10.1016/j.clinbiomech.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Cresswell AG. Responses of intra-abdominal pressure and abdominal muscle activity during dynamic trunk loading in man. Eur J Appl Physiol Occup Physiol. 1993;66:315–320. doi: 10.1007/BF00237775. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Richardson CA. Transversus abdominis and the superficial abdominal muscles are controlled independently in a postural task. Neurosci Lett. 1999;265:91–94. doi: 10.1016/s0304-3940(99)00216-5. [DOI] [PubMed] [Google Scholar]
- Anderson K, Behm DG. Trunk muscle activity increases with unstable squat movements. Can J Appl Physiol. 2005;30:33–45. doi: 10.1139/h05-103. [DOI] [PubMed] [Google Scholar]
- Granata KP, Orishimo KF, Sanford AH. Trunk muscle coactivation in preparation for sudden load. J Electromyogr Kinesiol. 2001;11:247–254. doi: 10.1016/s1050-6411(01)00003-7. [DOI] [PubMed] [Google Scholar]
- Huang QM, Andersson E, Thorstensson A. Intramuscular myoelectric activity and selective coactivation of trunk muscles during lateral flexion with and without load. Spine. 2001;26:1465–1472. doi: 10.1097/00007632-200107010-00017. [DOI] [PubMed] [Google Scholar]
- Wilder DG, Aleksiev AR, Magnusson ML, Pope MH, Spratt KF, Goel VK. Muscular response to sudden load: a tool to evaluate fatigue and rehabilitation. Spine. 1996;21:2628–2639. doi: 10.1097/00007632-199611150-00013. [DOI] [PubMed] [Google Scholar]
- Ferreira PH, Ferreira ML, Hodges PW. Changes in recruitment of the abdominal muscles in people with low back pain. Spine. 2004;29:2560–2566. doi: 10.1097/01.brs.0000144410.89182.f9. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Richardson CA. Inefficient muscular stabilization of the lumbar spine associated with low back pain. Spine. 1996;21:2640–2650. doi: 10.1097/00007632-199611150-00014. [DOI] [PubMed] [Google Scholar]
- McGill SM, Juker D, Kropf P. Appropriately placed EMG surface electrodes reflect deep muscle activity (psoas, quadratus lumborum, abdominal wall) in the lumbar spine. J Biomech. 1996;29:1503–1507. doi: 10.1016/0021-9290(96)84547-7. [DOI] [PubMed] [Google Scholar]
- Marshall P, Murphy B. The validity and reliability of surface EMG to assess the neuromuscular response of the abdominal muscles to rapid limb movement. J Electromyogr Kinesiol. 2003;13:477–489. doi: 10.1016/s1050-6411(03)00027-0. [DOI] [PubMed] [Google Scholar]
- Dankaerts W, O'Sullivan PB, Burnett AF, Straker LM, Danneels LA. Reliability of EMG measurements for trunk muscles during maximal and sub-maximal voluntary isometric contractions in healthy controls and CLBP patients. J Electromyogr Kinesiol. 2004;14:333–342. doi: 10.1016/j.jelekin.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Cresswell AG, Thorstensson A. Intra-abdominal pressure response to multi-directional support-surface translation. Gait Posture. 2004;20:163–170. doi: 10.1016/j.gaitpost.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Cholewicki J, Simons APD, Radebold A. Effects of external trunk loads on lumbar spine stability. J Biomech. 2000;33:1377–1385. doi: 10.1016/s0021-9290(00)00118-4. [DOI] [PubMed] [Google Scholar]
- Critchley D. Instructing pelvic floor contraction facilitates transversus abdominis thickness increase during low-abdominal hollowing. Physiother Res Int. 2002;7:65–75. doi: 10.1002/pri.243. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Cresswell AG, Daggfeldt K, Thorstensson A. In vivo measurement of the effect of intra-abdominal pressure on the human spine. J Biomech. 2001;34:347–353. doi: 10.1016/s0021-9290(00)00206-2. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Eriksson AEM, Shirley D, Gandevia SC. Intra-abdominal pressure increases stiffness of the lumbar spine. J Biomech. 2005;38:1873–1880. doi: 10.1016/j.jbiomech.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Urquhart DM, Barker PJ, Hodges PW, Story IH, Briggs CA. Regional morphology of the transversus abdominis and obliquus internus and externus abdominis muscles. Clin Biomech (Bristol, Avon) 2005;20:233–241. doi: 10.1016/j.clinbiomech.2004.11.007. [DOI] [PubMed] [Google Scholar]