Abstract
The transferable macrolides-lincosamides-streptogramin B (MLS) resistance determinant of clinical isolates of Clostridium difficile, designated ermZ, has been shown to share homology with ermB, which is associated with Staphylococcus aureus transposon Tn551. Homology within Tn551 was confined to less than or equal to 1.3 kilobases, whereas no homology could be demonstrated between Tn551 sequences external to ermB and MLS-resistant C. difficile. Transfer of ermZ from C. difficile to S. aureus was achieved by means of the filter mating technique, suggesting that (conjugative?) intergeneric exchange between clostridia and staphylococci may also occur in nature. S. aureus transcipients were shown to contain additional DNA from C. difficile besides ermZ. This additional DNA appeared to be present in MLS-susceptible C. difficile and might form part of an as yet undemonstrated insertion sequence element associated with ermZ of resistant strains.
Full text
PDF

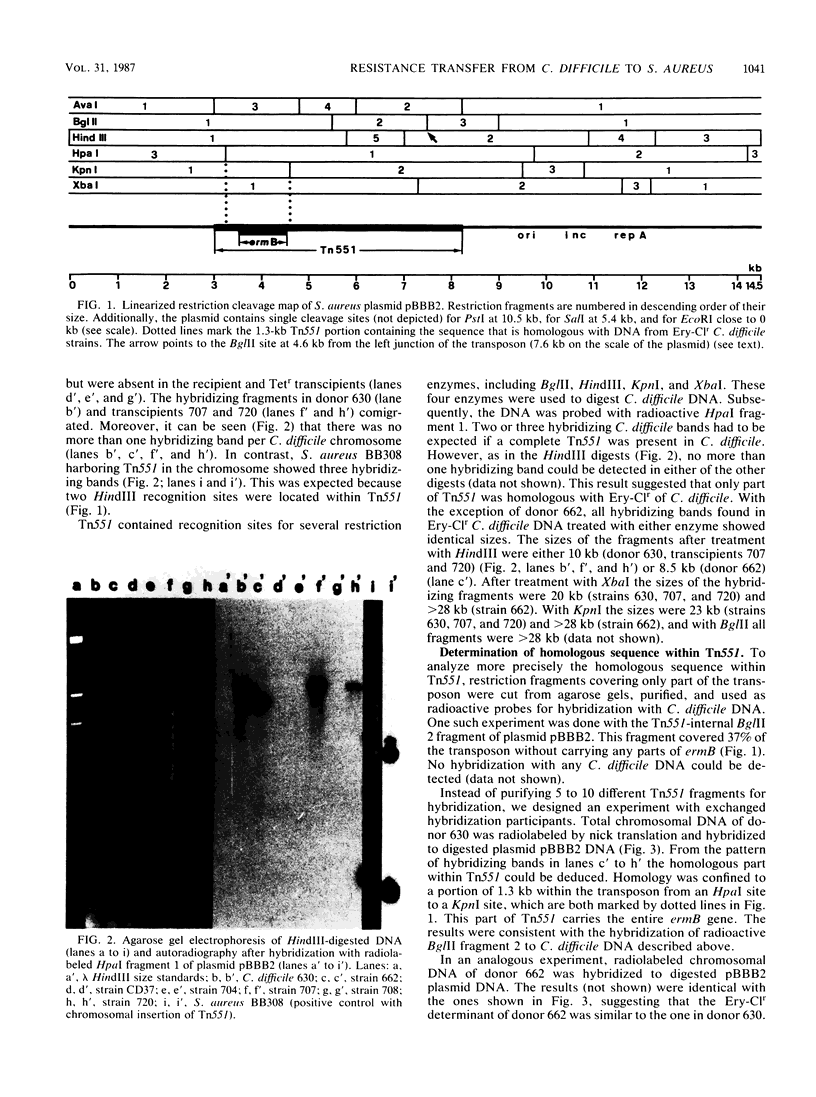




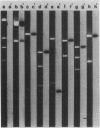
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett J. G., Chang T. W., Gurwith M., Gorbach S. L., Onderdonk A. B. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978 Mar 9;298(10):531–534. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- Berger-Bächi B. Increase in transduction efficiency of Tn551 mediated by the methicillin resistance marker. J Bacteriol. 1983 Apr;154(1):533–535. doi: 10.1128/jb.154.1.533-535.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger-Bächi B. Insertional inactivation of staphylococcal methicillin resistance by Tn551. J Bacteriol. 1983 Apr;154(1):479–487. doi: 10.1128/jb.154.1.479-487.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P., Ounissi H., Arthur M. Multiplicity of macrolide-lincosamide-streptogramin antibiotic resistance determinants. J Antimicrob Chemother. 1985 Jul;16 (Suppl A):91–100. doi: 10.1093/jac/16.suppl_a.91. [DOI] [PubMed] [Google Scholar]
- Dzink J., Bartlett J. G. In vitro susceptibility of Clostridium difficile isolates from patients with antibiotic-associated diarrhea or colitis. Antimicrob Agents Chemother. 1980 Apr;17(4):695–698. doi: 10.1128/aac.17.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund M. W., Poysky F. T., Reed S. M., Smith C. A. Bacteriophage and the toxigenicity of Clostridium botulinum type C. Science. 1971 Apr 30;172(3982):480–482. doi: 10.1126/science.172.3982.480. [DOI] [PubMed] [Google Scholar]
- Engel H. W., Soedirman N., Rost J. A., van Leeuwen W. J., van Embden J. D. Transferability of macrolide, lincomycin, and streptogramin resistances between group A, B, and D streptococci, Streptococcus pneumoniae, and Staphylococcus aureus. J Bacteriol. 1980 May;142(2):407–413. doi: 10.1128/jb.142.2.407-413.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A. E., Clewell D. B. Evidence for a chromosome-borne resistance transposon (Tn916) in Streptococcus faecalis that is capable of "conjugal" transfer in the absence of a conjugative plasmid. J Bacteriol. 1981 Jan;145(1):494–502. doi: 10.1128/jb.145.1.494-502.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George R. H., Symonds J. M., Dimock F., Brown J. D., Arabi Y., Shinagawa N., Keighley M. R., Alexander-Williams J., Burdon D. W. Identification of Clostridium difficile as a cause of pseudomembranous colitis. Br Med J. 1978 Mar 18;1(6114):695–695. doi: 10.1136/bmj.1.6114.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintermann G., Fischer H. M., Crameri R., Hütter R. Simple procedure for distinguishing CCC, OC, and L forms of plasmid DNA by agarose gel electrophoresis. Plasmid. 1981 May;5(3):371–373. doi: 10.1016/0147-619x(81)90012-3. [DOI] [PubMed] [Google Scholar]
- Hächler H., Kayser F. H., Berger-Bächi B. Homology of a transferable tetracycline resistance determinant of Clostridium difficile with Streptococcus (Enterococcus) faecalis transposon Tn916. Antimicrob Agents Chemother. 1987 Jul;31(7):1033–1038. doi: 10.1128/aac.31.7.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionesco H. Transfert de la résistance à la tétracycline chez Clostridium difficile. Ann Microbiol (Paris) 1980 Mar-Apr;131A(2):171–179. [PubMed] [Google Scholar]
- Kieser T. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid. 1984 Jul;12(1):19–36. doi: 10.1016/0147-619x(84)90063-5. [DOI] [PubMed] [Google Scholar]
- Larson H. E., Price A. B., Honour P., Borriello S. P. Clostridium difficile and the aetiology of pseudomembranous colitis. Lancet. 1978 May 20;1(8073):1063–1066. doi: 10.1016/s0140-6736(78)90912-1. [DOI] [PubMed] [Google Scholar]
- Mays T. D., Smith C. J., Welch R. A., Delfini C., Macrina F. L. Novel antibiotic resistance transfer in Bacteroides. Antimicrob Agents Chemother. 1982 Jan;21(1):110–118. doi: 10.1128/aac.21.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldrow L. L., Archibold E. R., Nunez-Montiel O. L., Sheehy R. J. Survey of the extrachromosomal gene pool of Clostridium difficile. J Clin Microbiol. 1982 Oct;16(4):637–640. doi: 10.1128/jcm.16.4.637-640.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E., Huwyler L., de Freire Bastos M. do C. Transposon Tn554: complete nucleotide sequence and isolation of transposition-defective and antibiotic-sensitive mutants. EMBO J. 1985 Dec 1;4(12):3357–3365. doi: 10.1002/j.1460-2075.1985.tb04089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Edelman I., Schwesinger M. D., Gruss A. D., Swanson E. C., Pattee P. A. Genetic translocation in Staphylococcus aureus. Proc Natl Acad Sci U S A. 1979 Jan;76(1):400–404. doi: 10.1073/pnas.76.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Murphy E. MLS-resistance determinants in Staphylococcus aureus and their molecular evolution. J Antimicrob Chemother. 1985 Jul;16 (Suppl A):101–110. doi: 10.1093/jac/16.suppl_a.101. [DOI] [PubMed] [Google Scholar]
- Perkins J. B., Youngman P. J. A physical and functional analysis of Tn917, a Streptococcus transposon in the Tn3 family that functions in Bacillus. Plasmid. 1984 Sep;12(2):119–138. doi: 10.1016/0147-619x(84)90058-1. [DOI] [PubMed] [Google Scholar]
- Sell T. L., Schaberg D. R., Fekety F. R. Bacteriophage and bacteriocin typing scheme for Clostridium difficile. J Clin Microbiol. 1983 Jun;17(6):1148–1152. doi: 10.1128/jcm.17.6.1148-1152.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. H., Clewell D. B. Complete nucleotide sequence of macrolide-lincosamide-streptogramin B-resistance transposon Tn917 in Streptococcus faecalis. J Bacteriol. 1985 Nov;164(2):782–796. doi: 10.1128/jb.164.2.782-796.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. J., Markowitz S. M., Macrina F. L. Transferable tetracycline resistance in Clostridium difficile. Antimicrob Agents Chemother. 1981 Jun;19(6):997–1003. doi: 10.1128/aac.19.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Uchiyama H., Weisblum B. N-Methyl transferase of Streptomyces erythraeus that confers resistance to the macrolide-lincosamide-streptogramin B antibiotics: amino acid sequence and its homology to cognate R-factor enzymes from pathogenic bacilli and cocci. Gene. 1985;38(1-3):103–110. doi: 10.1016/0378-1119(85)90208-2. [DOI] [PubMed] [Google Scholar]
- Weisblum B. Inducible resistance to macrolides, lincosamides and streptogramin type B antibiotics: the resistance phenotype, its biological diversity, and structural elements that regulate expression--a review. J Antimicrob Chemother. 1985 Jul;16 (Suppl A):63–90. doi: 10.1093/jac/16.suppl_a.63. [DOI] [PubMed] [Google Scholar]
- Welch R. A., Jones K. R., Macrina F. L. Transferable lincosamide-macrolide resistance in Bacteroides. Plasmid. 1979 Apr;2(2):261–268. doi: 10.1016/0147-619x(79)90044-1. [DOI] [PubMed] [Google Scholar]
- Wüst J., Hardegger U. Transferable resistance to clindamycin, erythromycin, and tetracycline in Clostridium difficile. Antimicrob Agents Chemother. 1983 May;23(5):784–786. doi: 10.1128/aac.23.5.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüst J., Sullivan N. M., Hardegger U., Wilkins T. D. Investigation of an outbreak of antibiotic-associated colitis by various typing methods. J Clin Microbiol. 1982 Dec;16(6):1096–1101. doi: 10.1128/jcm.16.6.1096-1101.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Solh N., Allignet J., Bismuth R., Buret B., Fouace J. M. Conjugative transfer of staphylococcal antibiotic resistance markers in the absence of detectable plasmid DNA. Antimicrob Agents Chemother. 1986 Jul;30(1):161–169. doi: 10.1128/aac.30.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]