Abstract
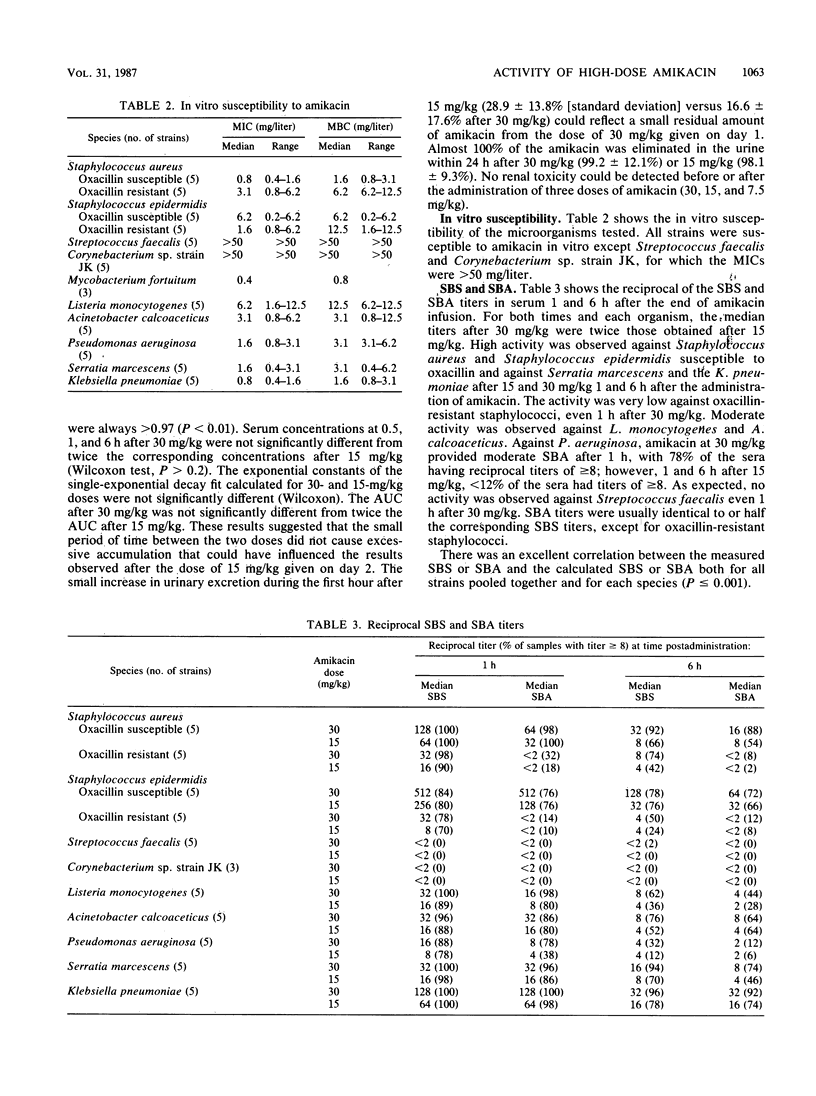
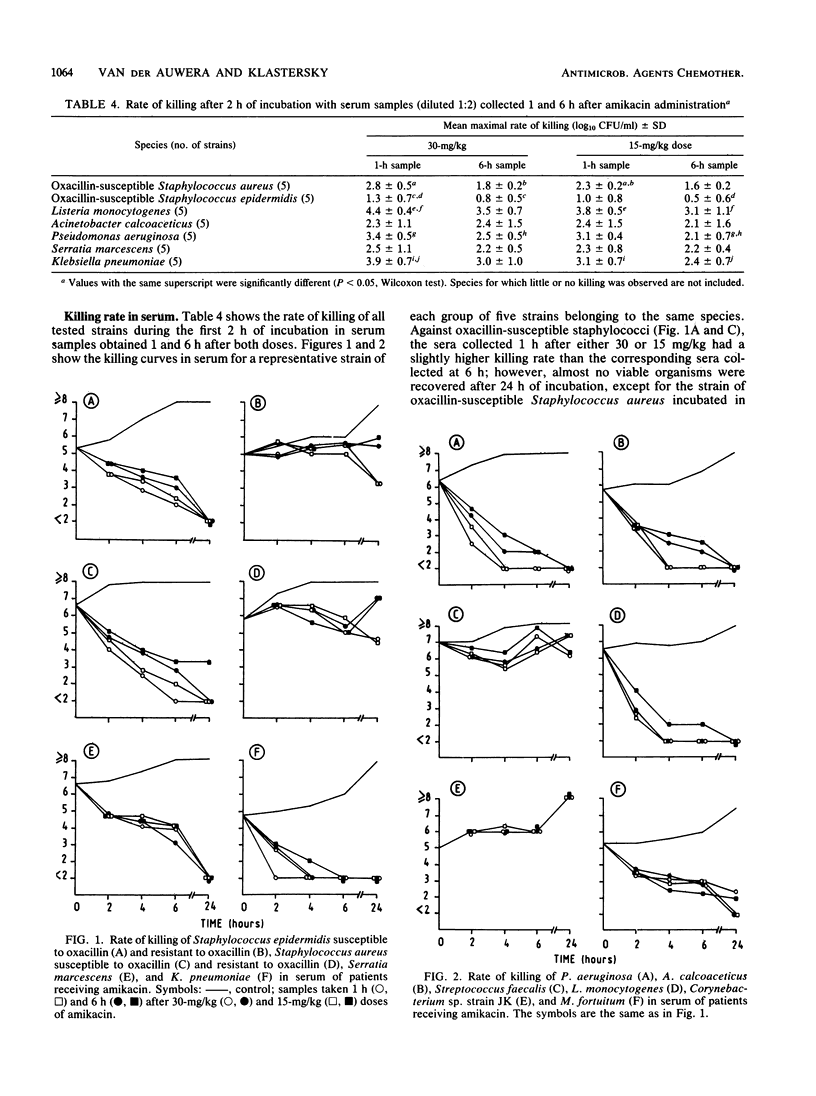
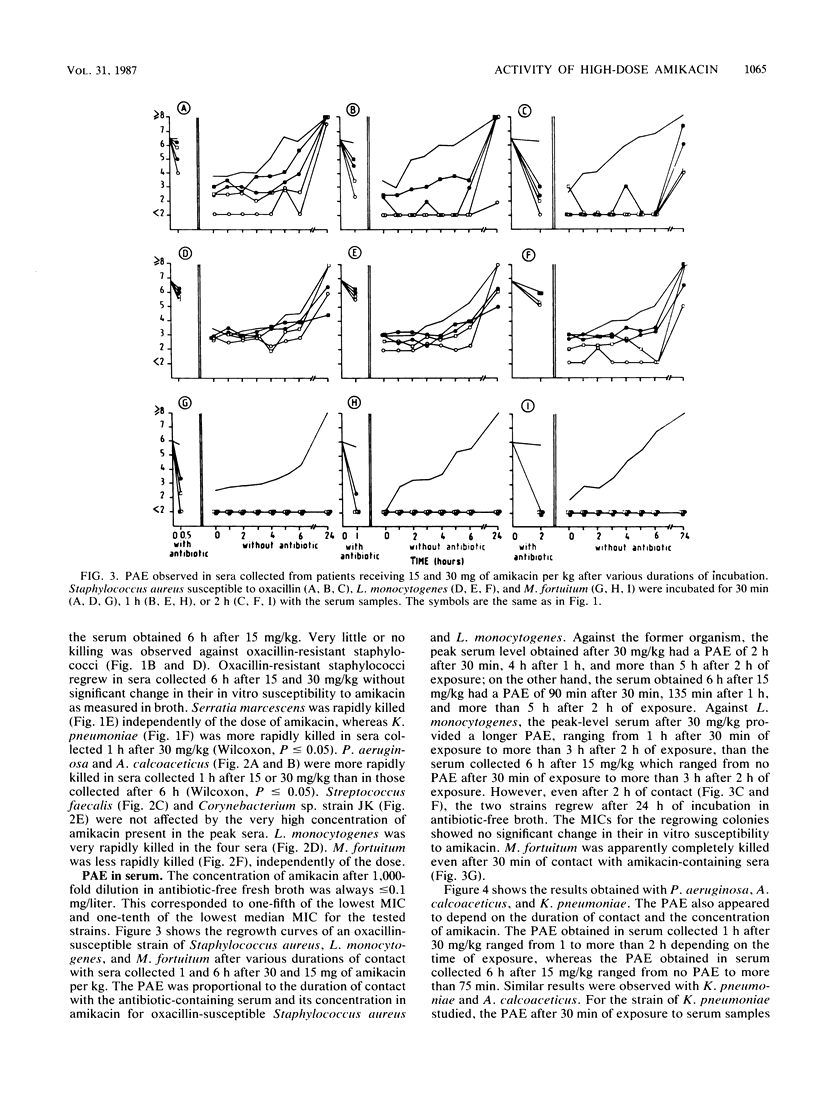
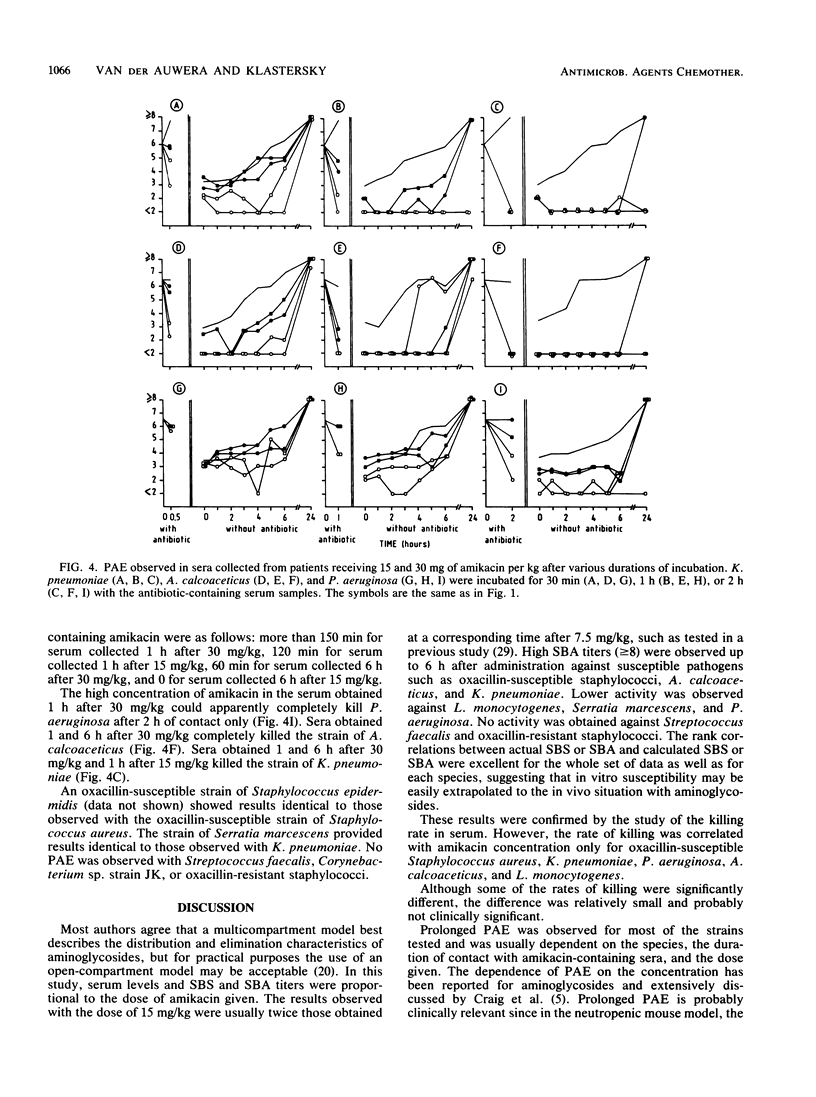
Ten patients received a 30-min infusion of amikacin (30 mg/kg) on day 1 and 15 mg/kg on day 2. Mean serum creatinine was 1.1 +/- 0.3 (standard deviation) mg/dl before and 1.0 +/- 0.3 mg/dl 3 days after the second infusion. Mean serum amikacin concentrations before, at the end of infusion, and 1, 6, 12, and 24 h after 30 and 15 mg/kg were 0, 157, 79, 31, 16, 5, 5, 85, 51, 19, 12, and 5 mg/liter, respectively. Five strains each of Staphylococcus aureus, Staphylococcus epidermidis susceptible and resistant to oxacillin, Streptococcus (Enterococcus) faecalis, corynebacterium sp. strain JK, Listeria monocytogenes, Mycobacterium fortuitum (three strains), Klebsiella pneumoniae, Serratia marcescens, Acinetobacter calcoaceticus, and Pseudomonas aeruginosa were tested. Serum bactericidal activities (SBAs) were greater than or equal to 1:8 in greater than or equal to 80% of the sera 1 and 6 h after 30 mg/kg and in greater than or equal to 60% of the sera 1 and 6 h after 15 mg/kg against Staphylococcus aureus and Staphylococcus epidermidis susceptible to oxacillin, A. calcoaceticus, and K. pneumoniae. L. monocytogenes, Serratia marcescens, and P. aeruginosa had lower SBAs. Very low or no activity was observed against oxacillin-resistant staphylococci and Streptococcus faecalis. The study of the killing rate in serum confirmed these results. Postantibiotic effect was studied by incubating a strain from each species in serum samples obtained 1 and 6 h after both regimens for 0.5, 1, or 2 h. The duration of postantibiotic effect depended on the duration of contact and the concentration of amikacin for the following organisms: oxacillin-susceptible staphylococci, L. monocytogenes, P. aeruginosa, A. calcoaceticus, K. pneumoniae, and Serratia marcescens. M. fortuitum was killed after 30 min of contact. No postantibiotic effect was observed with Streptococcus faecalis, Corynebacterium sp. strain JK, or oxacillin-resistant staphylococci. Amikacin at 30 mg/kg provided high levels and SBAs against susceptible pathogens. Prolonged postantibiotic effects were observed. No signs of nephrotoxicity occurred.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett W. M., Plamp C. E., Gilbert D. N., Parker R. A., Porter G. A. The influence of dosage regimen on experimental gentamicin nephrotoxicity: dissociation of peak serum levels from renal failure. J Infect Dis. 1979 Oct;140(4):576–580. doi: 10.1093/infdis/140.4.576. [DOI] [PubMed] [Google Scholar]
- Bergeron M. G., Bergeron Y. Influence of endotoxin on the intrarenal distribution of gentamicin, netilmicin, tobramycin, amikacin, and cephalothin. Antimicrob Agents Chemother. 1986 Jan;29(1):7–12. doi: 10.1128/aac.29.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron M. G. Therapeutic potential of high renal levels of aminoglycosides in pyelonephritis. J Antimicrob Chemother. 1985 Jan;15(1):4–8. doi: 10.1093/jac/15.1.4. [DOI] [PubMed] [Google Scholar]
- Bundtzen R. W., Gerber A. U., Cohn D. L., Craig W. A. Postantibiotic suppression of bacterial growth. Rev Infect Dis. 1981 Jan-Feb;3(1):28–37. doi: 10.1093/clinids/3.1.28. [DOI] [PubMed] [Google Scholar]
- Drake T. A., Hackbarth C. J., Sande M. A. Value of serum tests in combined drug therapy of endocarditis. Antimicrob Agents Chemother. 1983 Nov;24(5):653–657. doi: 10.1128/aac.24.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley R. S., Fortner C. L., deJongh C. A., Wade J. C., Newman K. A., Caplan E., Britten J., Wiernik P. H., Schimpff S. C. Comparison of standard versus pharmacokinetically adjusted amikacin dosing in granulocytopenic cancer patients. Antimicrob Agents Chemother. 1982 Aug;22(2):193–197. doi: 10.1128/aac.22.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber A. U., Craig W. A., Brugger H. P., Feller C., Vastola A. P., Brandel J. Impact of dosing intervals on activity of gentamicin and ticarcillin against Pseudomonas aeruginosa in granulocytopenic mice. J Infect Dis. 1983 May;147(5):910–917. doi: 10.1093/infdis/147.5.910. [DOI] [PubMed] [Google Scholar]
- Giuliano R. A., Verpooten G. A., Verbist L., Wedeen R. P., De Broe M. E. In vivo uptake kinetics of aminoglycosides in the kidney cortex of rats. J Pharmacol Exp Ther. 1986 Feb;236(2):470–475. [PubMed] [Google Scholar]
- Jolley M. E., Stroupe S. D., Wang C. H., Panas H. N., Keegan C. L., Schmidt R. L., Schwenzer K. S. Fluorescence polarization immunoassay. I. Monitoring aminoglycoside antibiotics in serum and plasma. Clin Chem. 1981 Jul;27(7):1190–1197. [PubMed] [Google Scholar]
- Kluge R. M., Standiford H. C., Tatem B., Young V. M., Schimpff S. C., Greene W. H., Calia F. M., Hornick R. B. The carbenicillin-gentamicin combination against Pseudomonas aeruginosa. Correlation of effect with gentamicin sensitivity. Ann Intern Med. 1974 Nov;81(5):584–587. doi: 10.7326/0003-4819-81-5-584. [DOI] [PubMed] [Google Scholar]
- Laurent G., Maldague P., Carlier M. B., Tulkens P. M. Increased renal DNA synthesis in vivo after administration of low doses of gentamicin to rats. Antimicrob Agents Chemother. 1983 Oct;24(4):586–593. doi: 10.1128/aac.24.4.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. D., Smith C. R., Lietman P. S. Association of aminoglycoside plasma levels with therapeutic outcome in gram-negative pneumonia. Am J Med. 1984 Oct;77(4):657–662. doi: 10.1016/0002-9343(84)90358-9. [DOI] [PubMed] [Google Scholar]
- Moore R. D., Smith C. R., Lietman P. S. The association of aminoglycoside plasma levels with mortality in patients with gram-negative bacteremia. J Infect Dis. 1984 Mar;149(3):443–448. doi: 10.1093/infdis/149.3.443. [DOI] [PubMed] [Google Scholar]
- Pearson R. D., Steigbigel R. T., Davis H. T., Chapman S. W. Method of reliable determination of minimal lethal antibiotic concentrations. Antimicrob Agents Chemother. 1980 Nov;18(5):699–708. doi: 10.1128/aac.18.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechere J. C., Dugal R. Clinical pharmacokinetics of aminoglycoside antibiotics. Clin Pharmacokinet. 1979 May-Jun;4(3):170–199. doi: 10.2165/00003088-197904030-00002. [DOI] [PubMed] [Google Scholar]
- Reiner N. E., Bloxham D. D., Thompson W. L. Nephrotoxicity of gentamicin and tobramycin given once daily or continuously in dogs. J Antimicrob Chemother. 1978 May;4 (Suppl A):85–101. doi: 10.1093/jac/4.suppl_a.85. [DOI] [PubMed] [Google Scholar]
- Reller L. B., Stratton C. W. Serum dilution test for bactericidal activity. II. Standardization and correlation with antimicrobial assays and susceptibility tests. J Infect Dis. 1977 Aug;136(2):196–204. doi: 10.1093/infdis/136.2.196. [DOI] [PubMed] [Google Scholar]
- Sanders C. C. Novel resistance selected by the new expanded-spectrum cephalosporins: a concern. J Infect Dis. 1983 Mar;147(3):585–589. doi: 10.1093/infdis/147.3.585. [DOI] [PubMed] [Google Scholar]
- Sculier J. P., Klastersky J. Significance of serum bactericidal activity in gram-negative bacillary bacteremia in patients with and without granulocytopenia. Am J Med. 1984 Mar;76(3):429–435. doi: 10.1016/0002-9343(84)90662-4. [DOI] [PubMed] [Google Scholar]
- Sculier J. P., Weerts D., Klastersky J. Causes of death in febrile granulocytopenic cancer patients receiving empiric antibiotic therapy. Eur J Cancer Clin Oncol. 1984 Jan;20(1):55–60. doi: 10.1016/0277-5379(84)90034-8. [DOI] [PubMed] [Google Scholar]
- Van der Auwera P. Interaction of gentamicin, dibekacin, netilmicin and amikacin with various penicillins, cephalosporins, minocycline and new fluoro-quinolones against Enterobacteriaceae and Pseudomonas aeruginosa. J Antimicrob Chemother. 1985 Nov;16(5):581–587. doi: 10.1093/jac/16.5.581. [DOI] [PubMed] [Google Scholar]
- Van der Auwera P., Klastersky J., Lieppe S., Husson M., Lauzon D., Lopez A. P. Bactericidal activity and killing rate of serum from volunteers receiving pefloxacin alone or in combination with amikacin. Antimicrob Agents Chemother. 1986 Feb;29(2):230–234. doi: 10.1128/aac.29.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein M. P., Stratton C. W., Ackley A., Hawley H. B., Robinson P. A., Fisher B. D., Alcid D. V., Stephens D. S., Reller L. B. Multicenter collaborative evaluation of a standardized serum bactericidal test as a prognostic indicator in infective endocarditis. Am J Med. 1985 Feb;78(2):262–269. doi: 10.1016/0002-9343(85)90436-x. [DOI] [PubMed] [Google Scholar]
- Wolfson J. S., Swartz M. N. Drug therapy. Serum bactericidal activity as a monitor of antibiotic therapy. N Engl J Med. 1985 Apr 11;312(15):968–975. doi: 10.1056/NEJM198504113121507. [DOI] [PubMed] [Google Scholar]


