Abstract
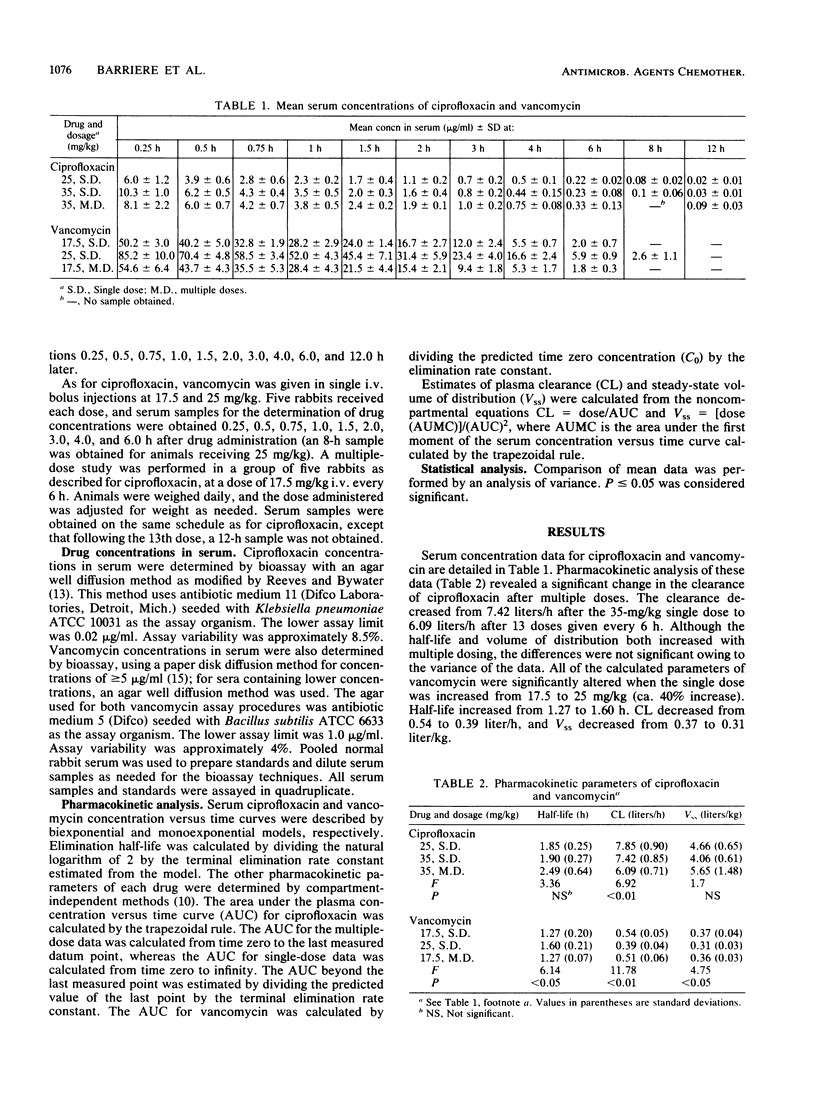
The pharmacokinetic disposition of vancomycin and ciprofloxacin was assessed in rabbits before the efficacy of these compounds in experimental staphylococcal endocarditis was compared. Ciprofloxacin was given in single intravenous bolus doses of 25 and 35 mg/kg and also in a multiple-dose regimen of 35 mg/kg every 6 h. Vancomycin was given in a similar manner in single doses of 17.5 and 25 mg/kg and in a multiple-dose regimen of 17.5 mg/kg every 6 h. Serum was sampled frequently after injections and analyzed by microbiologic assay for drug concentration. The pharmacokinetic parameters of clearance and steady-state volume of distribution were calculated by compartment-independent methods. These studies revealed that clearance of ciprofloxacin was reduced significantly after multiple doses (7.42 +/- 0.85 [standard deviation] versus 6.09 +/- 0.71 liters/h, P less than 0.01). Although the half-life and volume of distribution increased after multiple dosing, the differences were not statistically significant. The disposition of vancomycin following single doses was significantly altered after the 25-mg/kg dose compared with the 17.5-mg/kg dose. Half-life, clearance, and volume of distribution changed from 1.27 +/- 0.2 to 1.60 +/- 0.21 h (P less than 0.05), 0.54 +/- 0.05 to 0.39 +/- 0.04 liters/h (P less than 0.01), and 0.37 +/- 0.04 to 0.31 +/- 0.03 liters/kg (P less than 0.05), respectively. The disposition of ciprofloxacin was not altered with increases in dose size, and the disposition of vancomycin was not altered after multiple doses. If such alterations in the pharmacokinetic disposition of antimicrobial agents are unanticipated, the higher and more prolonged than expected serum concentrations may have an effect on the outcome of experimental infections.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronoff G. E., Kenner C. H., Sloan R. S., Pottratz S. T. Multiple-dose ciprofloxacin kinetics in normal subjects. Clin Pharmacol Ther. 1984 Sep;36(3):384–388. doi: 10.1038/clpt.1984.192. [DOI] [PubMed] [Google Scholar]
- Bayer A. S., Lam K. Efficacy of vancomycin plus rifampin in experimental aortic-valve endocarditis due to methicillin-resistant Staphylococcus aureus: in vitro-in vivo correlations. J Infect Dis. 1985 Jan;151(1):157–165. doi: 10.1093/infdis/151.1.157. [DOI] [PubMed] [Google Scholar]
- Bergan T., Delin C., Johansen S., Kolstad I. M., Nord C. E., Thorsteinsson S. B. Pharmacokinetics of ciprofloxacin and effect of repeated dosage on salivary and fecal microflora. Antimicrob Agents Chemother. 1986 Feb;29(2):298–302. doi: 10.1128/aac.29.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumfitt W., Franklin I., Grady D., Hamilton-Miller J. M., Iliffe A. Changes in the pharmacokinetics of ciprofloxacin and fecal flora during administration of a 7-day course to human volunteers. Antimicrob Agents Chemother. 1984 Nov;26(5):757–761. doi: 10.1128/aac.26.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter T. C., Hackbarth C. J., Chambers H. F., Sande M. A. Efficacy of ciprofloxacin for experimental endocarditis caused by methicillin-susceptible or -resistant strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Sep;30(3):382–384. doi: 10.1128/aac.30.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frimodt-Møller N., Bentzon M. W., Thomsen V. F. Experimental infection with Streptococcus pneumoniae in mice: correlation of in vitro activity and pharmacokinetic parameters with in vivo effect for 14 cephalosporins. J Infect Dis. 1986 Sep;154(3):511–517. doi: 10.1093/infdis/154.3.511. [DOI] [PubMed] [Google Scholar]
- Gilbert M., Boscia J. A., Kobasa W. D., Kaye D. Enoxacin compared with vancomycin for the treatment of experimental methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1986 Mar;29(3):461–463. doi: 10.1128/aac.29.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M. A., Moranchel A. H., Duran S., Pichardo A., Magana J. L., Painter B., Drusano G. L. Multiple-dose ciprofloxacin dose ranging and kinetics. Clin Pharmacol Ther. 1985 Jun;37(6):633–637. doi: 10.1038/clpt.1985.102. [DOI] [PubMed] [Google Scholar]
- Ingerman M. J., Pitsakis P. G., Rosenberg A. F., Levison M. E. The importance of pharmacodynamics in determining the dosing interval in therapy for experimental pseudomonas endocarditis in the rat. J Infect Dis. 1986 Apr;153(4):707–714. doi: 10.1093/infdis/153.4.707. [DOI] [PubMed] [Google Scholar]
- LeBel M., Vallée F., Bergeron M. G. Tissue penetration of ciprofloxacin after single and multiple doses. Antimicrob Agents Chemother. 1986 Mar;29(3):501–505. doi: 10.1128/aac.29.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden C. W., Shinners E. Ciprofloxacin as therapy for experimental osteomyelitis caused by Pseudomonas aeruginosa. J Infect Dis. 1985 Feb;151(2):291–294. doi: 10.1093/infdis/151.2.291. [DOI] [PubMed] [Google Scholar]
- Reeves D. S., Bywater M. J., Holt H. A., White L. O. In-vitro studies with ciprofloxacin, a new 4-quinolone compound. J Antimicrob Chemother. 1984 Apr;13(4):333–346. doi: 10.1093/jac/13.4.333. [DOI] [PubMed] [Google Scholar]
- Sabath L. D., Casey J. I., Ruch P. A., Stumpf L. L., Finland M. Rapid microassay of gentamicin, kanamycin, neomycin, streptomycin, and vancomycin in serum or plasma. J Lab Clin Med. 1971 Sep;78(3):457–463. [PubMed] [Google Scholar]
- Vazquez G. J., Archer G. L. Antibiotic therapy of experimental Staphylococcus epidermidis endocarditis. Antimicrob Agents Chemother. 1980 Feb;17(2):280–285. doi: 10.1128/aac.17.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Lockley R. M., Webberly M., Dent J. Pharmacokinetics of intravenously administered ciprofloxacin. Antimicrob Agents Chemother. 1984 Aug;26(2):208–210. doi: 10.1128/aac.26.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrob Agents Chemother. 1985 Oct;28(4):581–586. doi: 10.1128/aac.28.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]


