Abstract
LY146032, a cyclic lipopeptide antibiotic, is an inhibitor of cell wall peptidoglycan biosynthesis in gram-positive bacteria. Although LY146032 at relatively high concentrations inhibited the in vitro polymerization of UDP-linked sugar precursors, inhibition of cell wall formation in intact Staphylococcus aureus and Bacillus megaterium cells did not lead to the accumulation of UDP-N-acetyl-muramyl (MurNAc)-peptide(s). Experiments that measured formation of UDP-MurNAc-peptides revealed that LY146032 inhibited the formation of these nucleotide-linked intermediates. This antibiotic had a disruptive effect on membrane permeability as evidenced by the loss of intracellular potassium immediately after exposure to the drug. The lack of any major disruption of the phosphoenolpyruvate:sugar phosphotransferase system indicated that the membrane is not likely a lethal target for this antibiotic. The findings are consistent with a mechanism by which LY146032 inhibits the formation of precursor molecules utilized in peptidoglycan biosynthesis. The observed membrane effects likely result from transit of the inhibitor to its lethal target site.
Full text
PDF
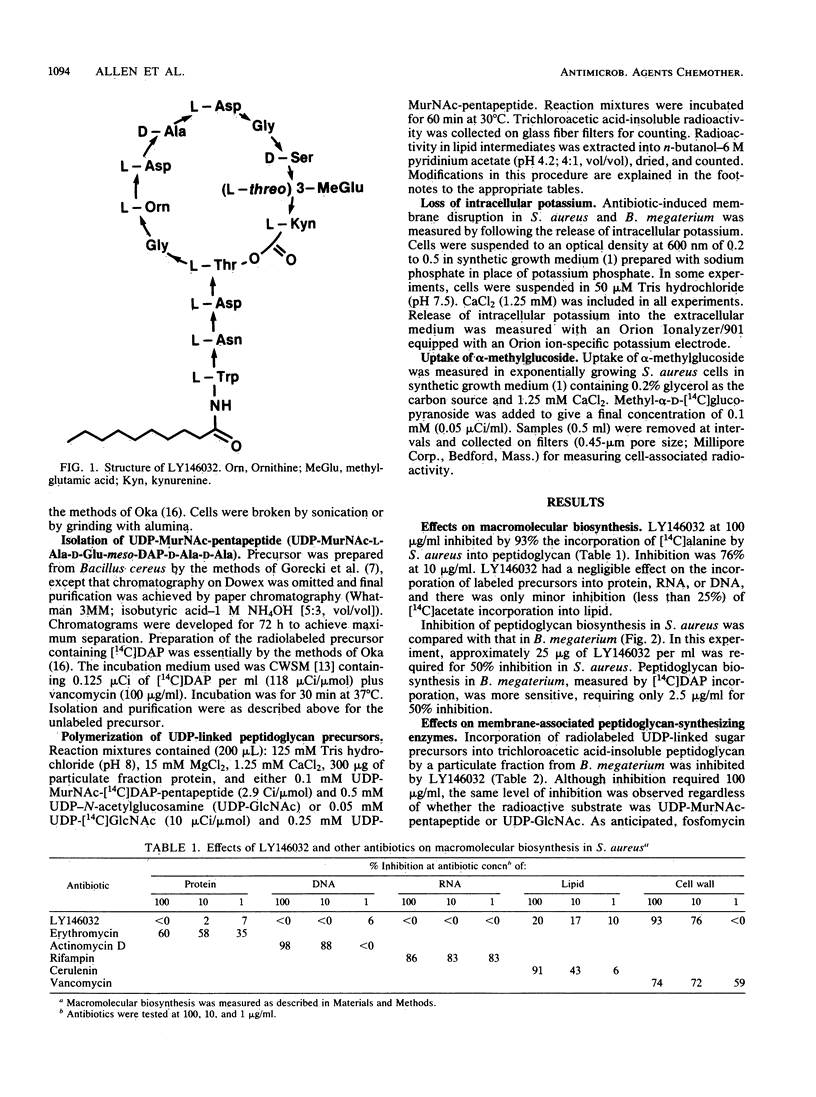
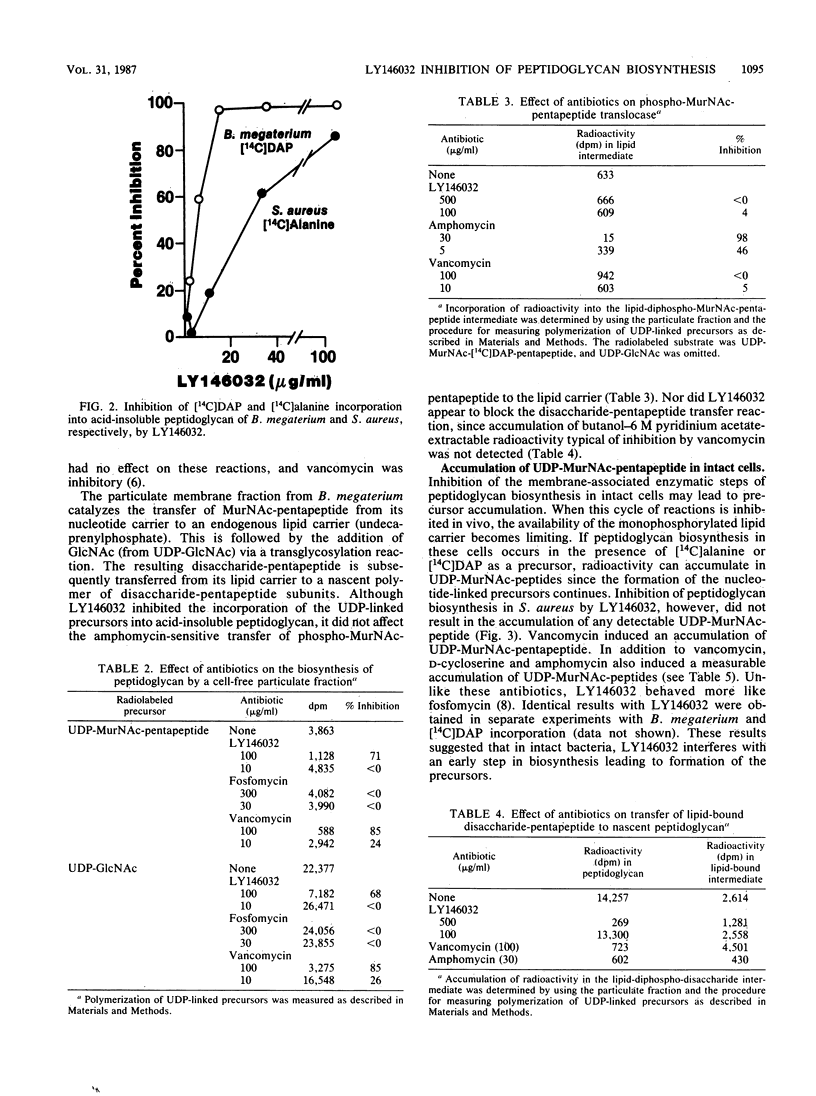
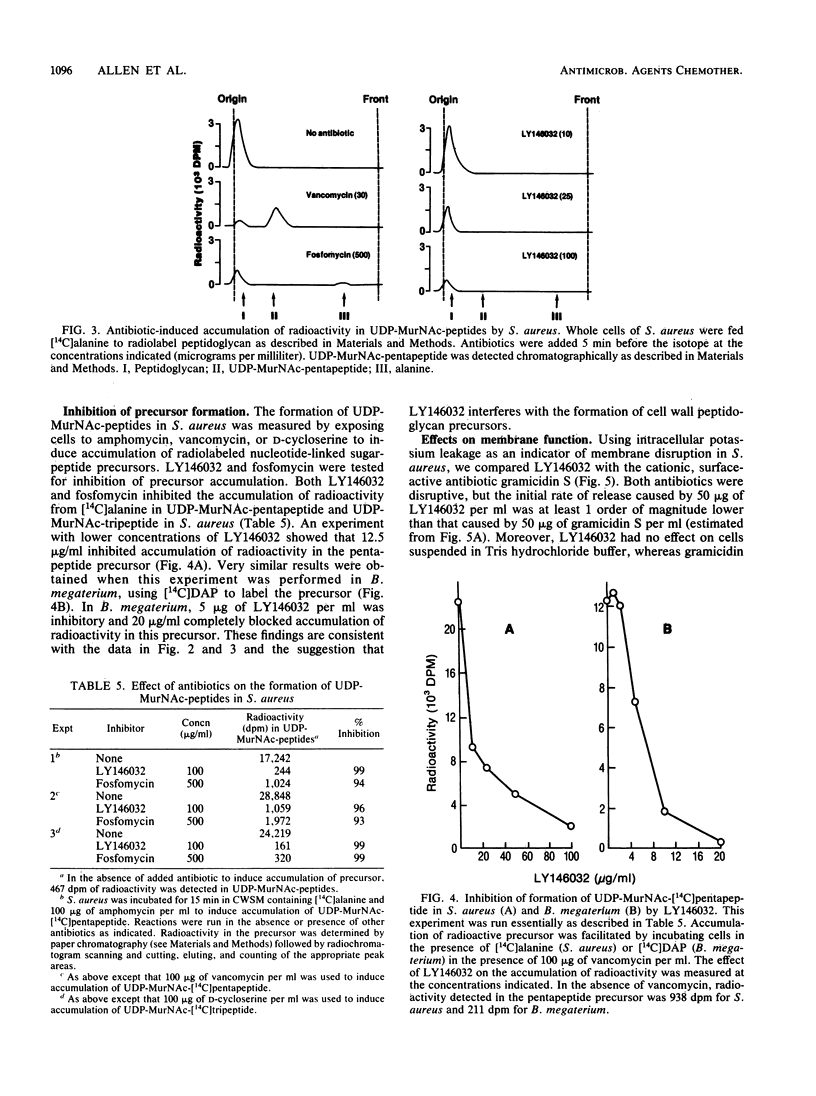
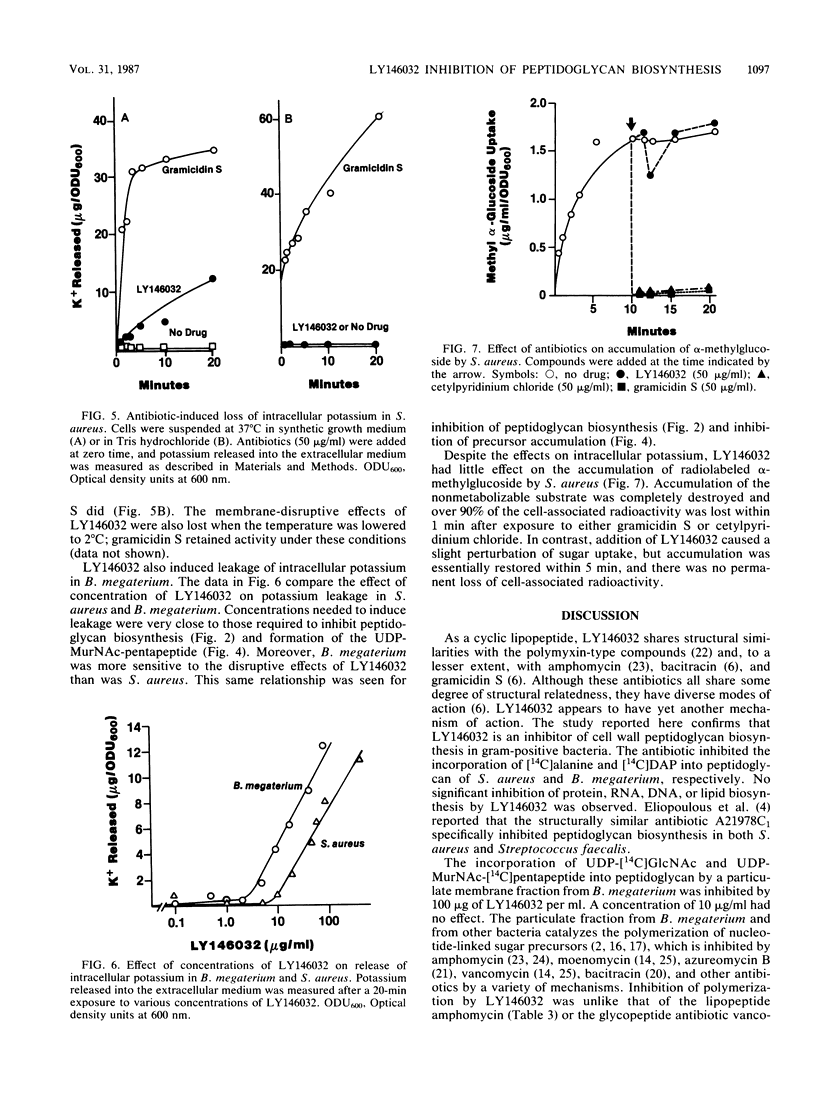


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON J. S., MATSUHASHI M., HASKIN M. A., STROMINGER J. L. LIPID-PHOSPHOACETYLMURAMYL-PENTAPEPTIDE AND LIPID-PHOSPHODISACCHARIDE-PENTAPEPTIDE: PRESUMED MEMBRANE TRANSPORT INTERMEDIATES IN CELL WALL SYNTHESIS. Proc Natl Acad Sci U S A. 1965 Apr;53:881–889. doi: 10.1073/pnas.53.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen N. E. Macrolide resistance in Staphylococcus aureus: induction of macrolide-resistant protein synthesis. Antimicrob Agents Chemother. 1977 Apr;11(4):661–668. doi: 10.1128/aac.11.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton F. R., Hall M. J., Hassall C. H., Lambert R. W., Lloyd W. J., Ringrose P. S. Phosphonopeptides as antibacterial agents: mechanism of action of alaphosphin. Antimicrob Agents Chemother. 1979 May;15(5):696–705. doi: 10.1128/aac.15.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos G. M., Thauvin C., Gerson B., Moellering R. C., Jr In vitro activity and mechanism of action of A21978C1, a novel cyclic lipopeptide antibiotic. Antimicrob Agents Chemother. 1985 Mar;27(3):357–362. doi: 10.1128/aac.27.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. A., Hays J. B. Initial characterization of hexose and hexitol phosphoenolpyruvate-dependent phosphotransferases of Staphylococcus aureus. J Bacteriol. 1977 Jun;130(3):991–999. doi: 10.1128/jb.130.3.991-999.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorecki M., Bar-Eli A., Burstein Y., Patchornik A., Chain E. B. Purification of D-alanine carboxypeptidase from Escherichia coli B on a penicillin-Sepharose column. Biochem J. 1975 Apr;147(1):131–137. doi: 10.1042/bj1470131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan F. M., Kahan J. S., Cassidy P. J., Kropp H. The mechanism of action of fosfomycin (phosphonomycin). Ann N Y Acad Sci. 1974 May 10;235(0):364–386. doi: 10.1111/j.1749-6632.1974.tb43277.x. [DOI] [PubMed] [Google Scholar]
- Kenig M., Abraham E. P. Antimicrobial activities and antagonists of bacilysin and anticapsin. J Gen Microbiol. 1976 May;94(1):37–45. doi: 10.1099/00221287-94-1-37. [DOI] [PubMed] [Google Scholar]
- Kenig M., Vandamme E., Abraham E. P. The mode of action of bacilysin and anticapsin and biochemical properties of bacilysin-resistant mutants. J Gen Microbiol. 1976 May;94(1):46–54. doi: 10.1099/00221287-94-1-46. [DOI] [PubMed] [Google Scholar]
- Lakey J. H., Lea E. J. The role of acyl chain character and other determinants on the bilayer activity of A21978C an acidic lipopeptide antibiotic. Biochim Biophys Acta. 1986 Jul 24;859(2):219–226. doi: 10.1016/0005-2736(86)90217-8. [DOI] [PubMed] [Google Scholar]
- Linnett P. E., Strominger J. L. Additional antibiotic inhibitors of peptidoglycan synthesis. Antimicrob Agents Chemother. 1973 Sep;4(3):231–236. doi: 10.1128/aac.4.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J., de Haan P. G. A simple method for following the fate of alanine-containing components in murein synthesis in Escherichia coli. Antonie Van Leeuwenhoek. 1971;37(4):537–552. doi: 10.1007/BF02218524. [DOI] [PubMed] [Google Scholar]
- Oka T. Mode of action of penicillins in vivo and in vitro in Bacillus megaterium. Antimicrob Agents Chemother. 1976 Oct;10(4):579–591. doi: 10.1128/aac.10.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P. E. Peptidoglycan synthesis in bacilli. I. Effect of temperature on the in vitro system from Bacillus megaterium and Bacillus stearothermophilus. Biochim Biophys Acta. 1971 May 18;237(2):239–254. [PubMed] [Google Scholar]
- Siewert G., Strominger J. L. Bacitracin: an inhibitor of the dephosphorylation of lipid pyrophosphate, an intermediate in the biosynthesis of the peptidoglycan of bacterial cell walls. Proc Natl Acad Sci U S A. 1967 Mar;57(3):767–773. doi: 10.1073/pnas.57.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiri-Nakagawa P., Tanaka Y., Oiwa R., Tanaka H., Omura S. Studies on bacterial cell wall inhibitors. VIII. Mode of action of a new antibiotic, azureomycin B, in Bacillus cereus T. J Antibiot (Tokyo) 1979 Oct;32(10):995–1001. doi: 10.7164/antibiotics.32.995. [DOI] [PubMed] [Google Scholar]
- Storm D. R., Rosenthal K. S., Swanson P. E. Polymyxin and related peptide antibiotics. Annu Rev Biochem. 1977;46:723–763. doi: 10.1146/annurev.bi.46.070177.003451. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Iwai Y., Oiwa R., Shinohara S., Shimizu S., Oka T., Omura S. Studies on bacterial cell wall inhibitors. II. Inhibition of peptidoglycan synthesis in vivo and in vitro by amphomycin. Biochim Biophys Acta. 1977 May 26;497(3):633–640. doi: 10.1016/0304-4165(77)90283-5. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Oiwa R., Matsukura S., Omura S. Amphomycin inhibits phospho-N-acetylmuramyl-pentapeptide translocase in peptidoglycan synthesis of Bacillus. Biochem Biophys Res Commun. 1979 Feb 14;86(3):902–908. doi: 10.1016/0006-291x(79)91797-2. [DOI] [PubMed] [Google Scholar]
- Van Heijenoort Y., Derrien M., Van Heijenoort J. Polymerization by transglycosylation in the biosynthesis of the peptidoglycan of Escherichia coli K 12 and its inhibition by antibiotics. FEBS Lett. 1978 May 1;89(1):141–144. doi: 10.1016/0014-5793(78)80540-7. [DOI] [PubMed] [Google Scholar]



