Abstract
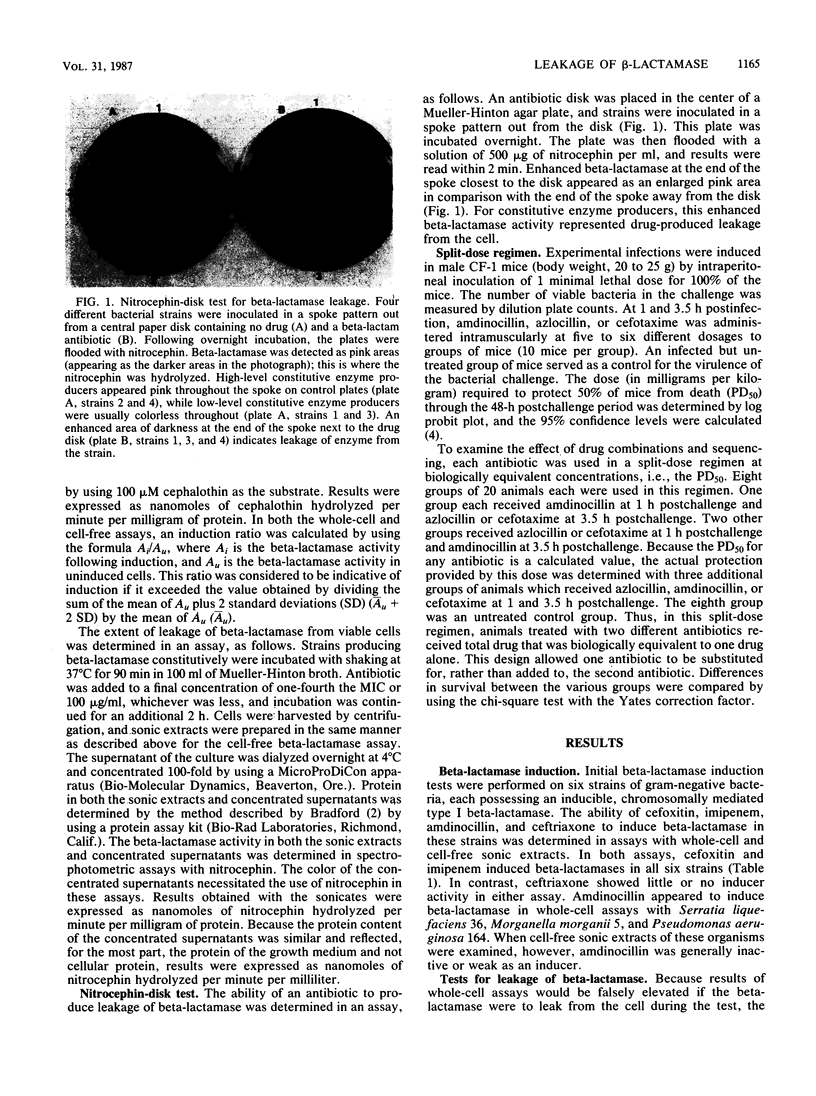
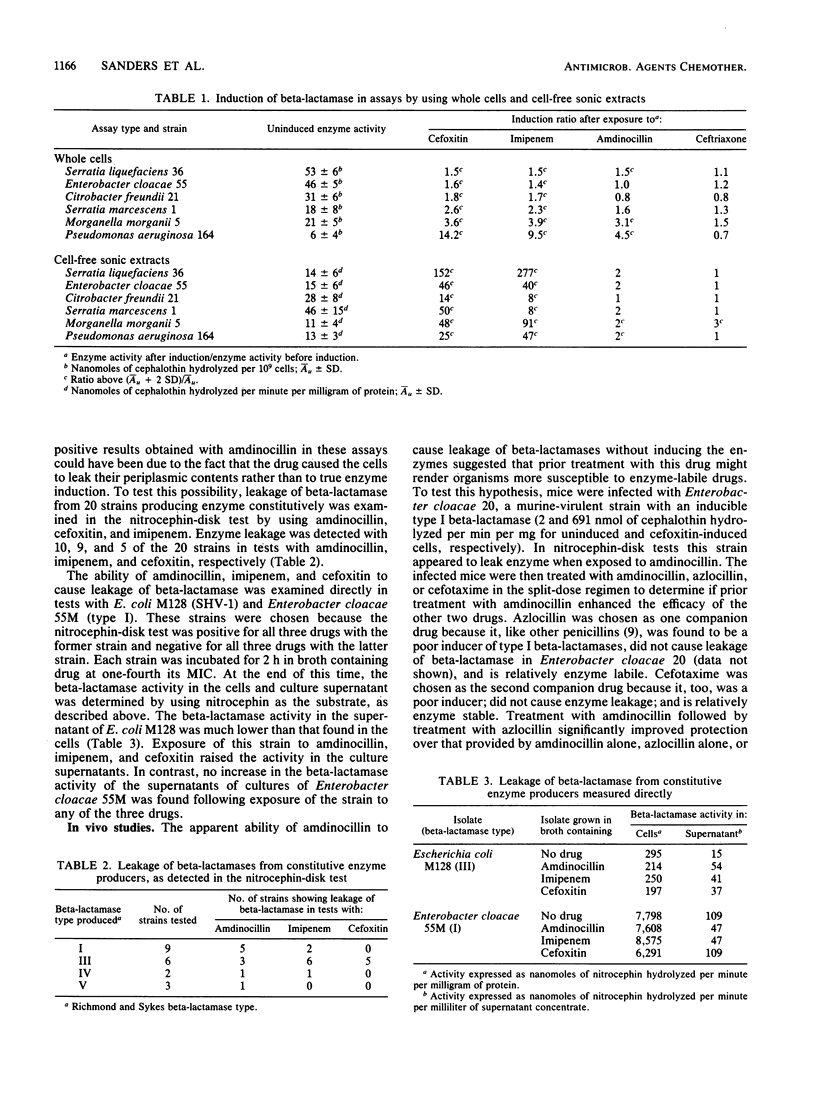
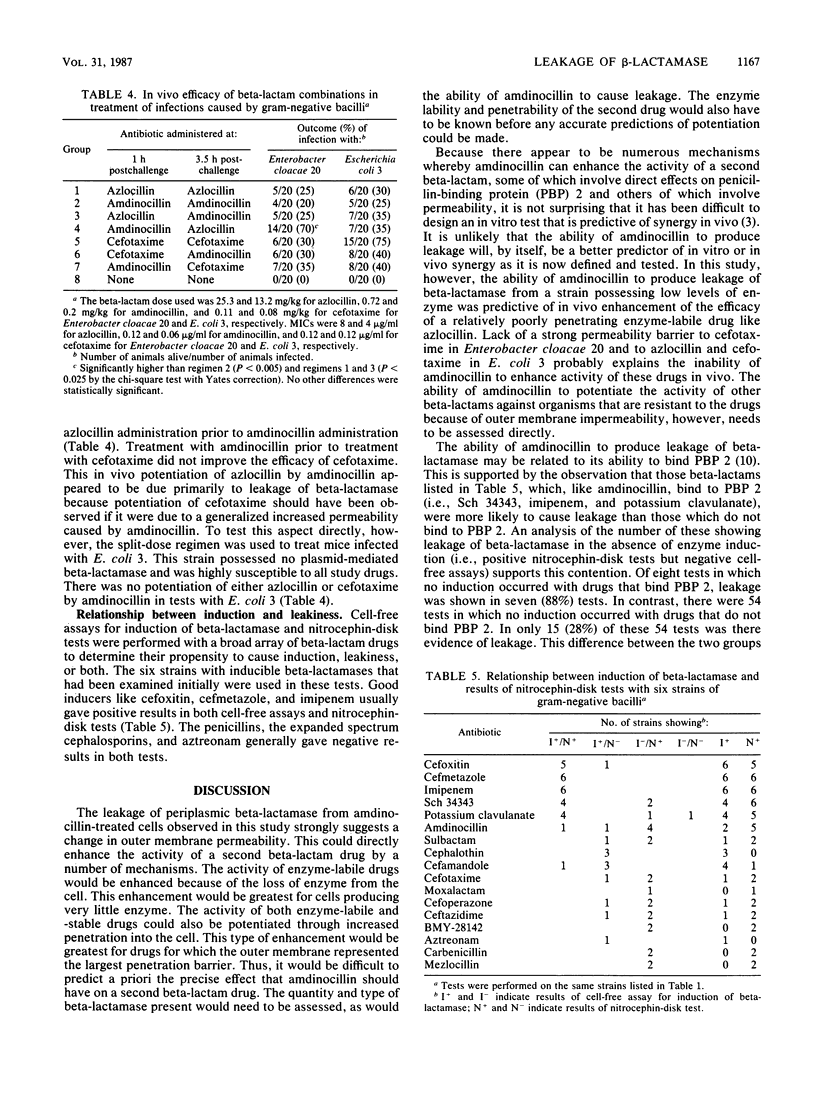
Discrepancies were observed between results of different beta-lactamase induction tests with amdinocillin, which appeared to be a strong inducer in whole-cell assays but a weak inducer in assays with cell-free sonic extracts. Results of a nitrocephin-disk test with constitutive beta-lactamase producers indicated that the positive results obtained in whole-cell assays were due to drug-produced leakage of enzyme from the cell and not to induction. Imipenem was also found to cause leakage of beta-lactamase from a similar number of constitutive enzyme producers, while cefoxitin was much less likely to cause leakage. A split-dose regimen was employed to treat mice infected with a strain of Enterobacter cloacae which appeared to leak enzyme on exposure to amdinocillin. Results indicated that prior treatment with amdinocillin significantly enhanced (P less than 0.025) the efficacy of azlocillin, an enzyme-labile drug, but did not affect the efficacy of cefotaxime, a relatively enzyme-stable drug. Conversely, prior treatment with amdinocillin did not potentiate the efficacy of either azlocillin or cefotaxime in the treatment of mice infected with an Escherichia coli strain that was highly susceptible to all three drugs. Thus, it appears that amdinocillin may potentiate the activity of other beta-lactam drugs not only by binding to a complementary penicillin-binding protein but also by causing leakage of beta-lactamase from the cell. This effect may be related to its ability to bind to penicillin-binding protein 2 and subsequently produce changes in outer membrane permeability.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakken J. S., Sanders C. C., Thomson K. S. Selective ceftazidime resistance in Escherichia coli: association with changes in outer membrane protein. J Infect Dis. 1987 Jun;155(6):1220–1225. doi: 10.1093/infdis/155.6.1220. [DOI] [PubMed] [Google Scholar]
- Barbour A. G., Mayer L. W., Spratt B. G. Mecillinam resistance in Escherichia coli: dissociation of growth inhibition and morphologic change. J Infect Dis. 1981 Jan;143(1):114–121. doi: 10.1093/infdis/143.1.114. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Isenberg H. D., Sampson-Scherer J., Cleeland R., Titsworth E., Beskid G., Christenson J. G., DeLorenzo W. F., Unowsky J. Correlation of the results of antibiotic synergy and susceptibility testing in vitro with results in experimental mouse infections. Crit Rev Microbiol. 1982;10(1):1–76. doi: 10.3109/10408418209113505. [DOI] [PubMed] [Google Scholar]
- Lund F., Tybring L. 6 -amidinopenicillanic acids--a new group of antibiotics. Nat New Biol. 1972 Apr 5;236(66):135–137. doi: 10.1038/newbio236135a0. [DOI] [PubMed] [Google Scholar]
- Matsuhashi S., Kamiryo T., Blumberg P. M., Linnett P., Willoughby E., Strominger J. L. Mechanism of action and development of resistance to a new amidino penicillin. J Bacteriol. 1974 Feb;117(2):578–587. doi: 10.1128/jb.117.2.578-587.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa H., Hayakawa K., Sato T., Imahori K. Characterization and genetic analysis of a mutant of Escherichia coli K-12 with rounded morphology. J Bacteriol. 1973 Jul;115(1):436–442. doi: 10.1128/jb.115.1.436-442.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Emergence of resistance to cefamandole: possible role of cefoxitin-inducible beta-lactamases. Antimicrob Agents Chemother. 1979 Jun;15(6):792–797. doi: 10.1128/aac.15.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Type I beta-lactamases of gram-negative bacteria: interactions with beta-lactam antibiotics. J Infect Dis. 1986 Nov;154(5):792–800. doi: 10.1093/infdis/154.5.792. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. The mechanism of action of mecillinam. J Antimicrob Chemother. 1977 Jul;3 (Suppl B):13–19. doi: 10.1093/jac/3.suppl_b.13. [DOI] [PubMed] [Google Scholar]
- Tamaki S., Matsuzawa H., Matsuhashi M. Cluster of mrdA and mrdB genes responsible for the rod shape and mecillinam sensitivity of Escherichia coli. J Bacteriol. 1980 Jan;141(1):52–57. doi: 10.1128/jb.141.1.52-57.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tybring L. Special aspects of laboratory investigations with mecillinam. J Antimicrob Chemother. 1977 Jul;3 (Suppl B):23–27. doi: 10.1093/jac/3.suppl_b.23. [DOI] [PubMed] [Google Scholar]
- Westling-Häggström B., Normark S. Genetic and physiological analysis of an envB spherelike mutant of Escherichia coli K-12 and characterization of its transductants. J Bacteriol. 1975 Jul;123(1):75–82. doi: 10.1128/jb.123.1.75-82.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]