Abstract
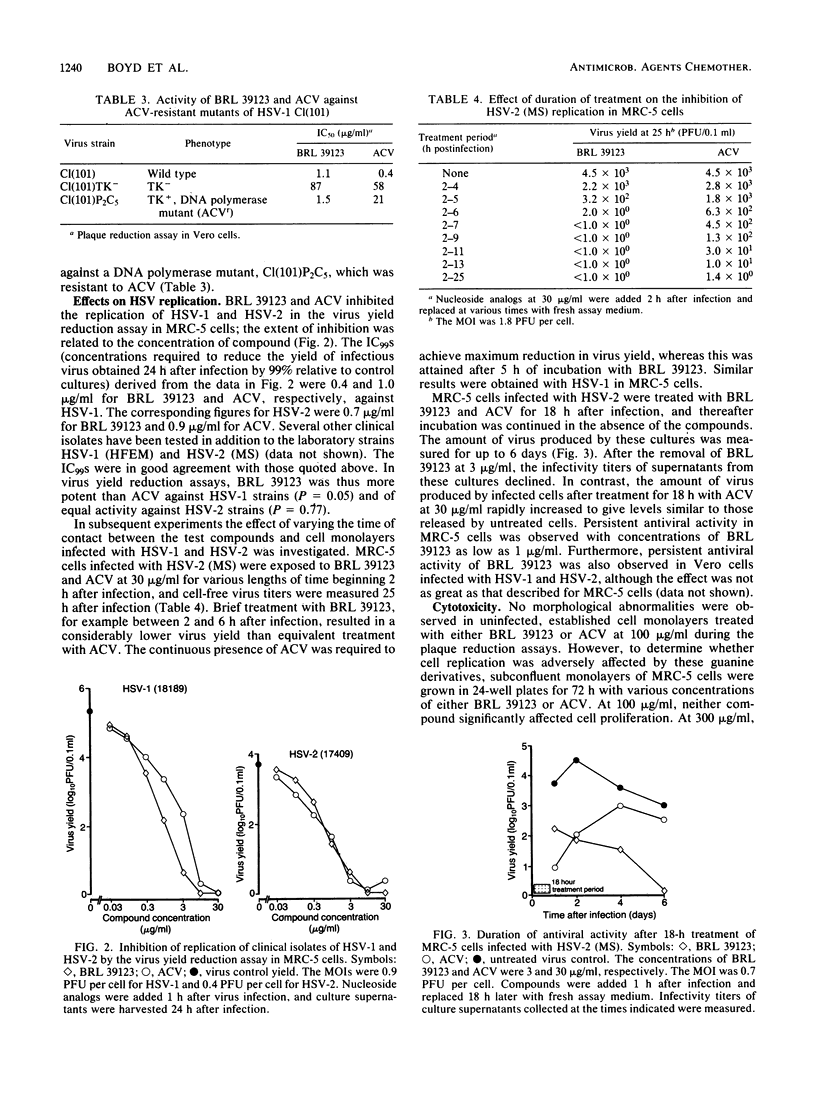
The activity of 9-(4-hydroxy-3-hydroxymethylbut-1-yl)guanine (BRL 39123) against several herpesviruses was compared with that of acyclovir (ACV). In plaque reduction tests with clinical isolates of herpes simplex virus type 1 (HSV-1), herpes simplex virus type 2 (HSV-2), and varicella-zoster virus, mean 50% inhibitory concentrations (IC50S) (n = number tested) for BRL 39123 were 0.4 (n = 17), 1.5 (n = 13), and 3.1 (n = 5) micrograms/ml, respectively. Corresponding IC50S for ACV were 0.2, 0.6, and 3.8 micrograms/ml. Cytomegalovirus was relatively resistant to BRL 39123 (IC50, 51 micrograms/ml), but equid herpesvirus 1, bovid herpesvirus 2, and felid herpesvirus 1 were susceptible (IC50S, 1.6, 1.2, and 0.9 micrograms/ml, respectively). BRL 39123 was inactive against an HSV-1 strain which does not express thymidine kinase activity, but a DNA polymerase mutant selected for resistance to ACV was sensitive to BRL 39123 (IC50, 1.5 micrograms/ml). In contrast to the results from plaque reduction tests, BRL 39123 was more active than ACV against HSV-1 and of equal activity against HSV-2 in virus yield reduction assays in MRC-5 cells. After treatment of HSV-infected cultures for short periods, BRL 39123 was considerably more effective than ACV at reducing virus replication, and furthermore, after removal of extracellular BRL 39123, virus replication remained depressed for long periods, whereas such persistent activity was not observed with ACV. Neither compound significantly affected MRC-5 cell replication at 100 micrograms/ml, but at 300 micrograms/ml BRL 39123 was more inhibitory than ACV.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton W. T., Canning L. F., Reynolds G. F., Tolman R. L., Karkas J. D., Liou R., Davies M. E., DeWitt C. M., Perry H. C., Field A. K. Synthesis and antiherpetic activity of (S)-, (R)-, and (+/-)-9-[(2,3-dihydroxy-1-propoxy)methyl]guanine, linear isomers of 2'-nor-2'-deoxyguanosine. J Med Chem. 1985 Jul;28(7):926–933. doi: 10.1021/jm00145a014. [DOI] [PubMed] [Google Scholar]
- Biron K. K., Elion G. B. In vitro susceptibility of varicella-zoster virus to acyclovir. Antimicrob Agents Chemother. 1980 Sep;18(3):443–447. doi: 10.1128/aac.18.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Grill S. P., Dutschman G. E., Nakayama K., Bastow K. F. Metabolism of 9-(1,3-dihydroxy-2-propoxymethyl)guanine, a new anti-herpes virus compound, in herpes simplex virus-infected cells. J Biol Chem. 1983 Oct 25;258(20):12460–12464. [PubMed] [Google Scholar]
- Cheng Y. C., Huang E. S., Lin J. C., Mar E. C., Pagano J. S., Dutschman G. E., Grill S. P. Unique spectrum of activity of 9-[(1,3-dihydroxy-2-propoxy)methyl]-guanine against herpesviruses in vitro and its mode of action against herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1983 May;80(9):2767–2770. doi: 10.1073/pnas.80.9.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby B. M., Shaw J. E., Elion G. B., Pagano J. S. Effect of acyclovir [9-(2-hydroxyethoxymethyl)guanine] on Epstein-Barr virus DNA replication. J Virol. 1980 May;34(2):560–568. doi: 10.1128/jvi.34.2.560-568.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P., Bauer D. J. Relative potencies of anti-herpes compounds. Ann N Y Acad Sci. 1977 Mar 4;284:49–59. doi: 10.1111/j.1749-6632.1977.tb21936.x. [DOI] [PubMed] [Google Scholar]
- Collins P., Oliver N. M. Comparison of the in vitro and in vivo antiherpes virus activities of the acyclic nucleosides, acyclovir (Zovirax) and 9-[(2-hydroxy-1-hydroxymethylethoxy)methyl]guanine (BWB759U). Antiviral Res. 1985 Jun;5(3):145–156. doi: 10.1016/0166-3542(85)90047-6. [DOI] [PubMed] [Google Scholar]
- Crumpacker C. S., Schnipper L. E., Zaia J. A., Levin M. J. Growth inhibition by acycloguanosine of herpesviruses isolated from human infections. Antimicrob Agents Chemother. 1979 May;15(5):642–645. doi: 10.1128/aac.15.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A. K., Davies M. E., DeWitt C., Perry H. C., Liou R., Germershausen J., Karkas J. D., Ashton W. T., Johnston D. B., Tolman R. L. 9-([2-hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine: a selective inhibitor of herpes group virus replication. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4139–4143. doi: 10.1073/pnas.80.13.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Darby G., Wildy P. Isolation and characterization of acyclovir-resistant mutants of herpes simplex virus. J Gen Virol. 1980 Jul;49(1):115–124. doi: 10.1099/0022-1317-49-1-115. [DOI] [PubMed] [Google Scholar]
- Larsson A., Alenius S., Johansson N. G., Oberg B. Antiherpetic activity and mechanism of action of 9-(4-hydroxybutyl)guanine. Antiviral Res. 1983 Aug;3(2):77–86. doi: 10.1016/0166-3542(83)90028-1. [DOI] [PubMed] [Google Scholar]
- Larsson A., Oberg B., Alenius S., Hagberg C. E., Johansson N. G., Lindborg B., Stening G. 9-(3,4-dihydroxybutyl)guanine, a new inhibitor of herpesvirus multiplication. Antimicrob Agents Chemother. 1983 May;23(5):664–670. doi: 10.1128/aac.23.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson A., Stenberg K., Ericson A. C., Haglund U., Yisak W. A., Johansson N. G., Oberg B., Datema R. Mode of action, toxicity, pharmacokinetics, and efficacy of some new antiherpesvirus guanosine analogs related to buciclovir. Antimicrob Agents Chemother. 1986 Oct;30(4):598–605. doi: 10.1128/aac.30.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar E. C., Cheng Y. C., Huang E. S. Effect of 9-(1,3-dihydroxy-2-propoxymethyl)guanine on human cytomegalovirus replication in vitro. Antimicrob Agents Chemother. 1983 Oct;24(4):518–521. doi: 10.1128/aac.24.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer H. J., Beauchamp L., de Miranda P., Elion G. B., Bauer D. J., Collins P. 9-(2-hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature. 1978 Apr 13;272(5654):583–585. doi: 10.1038/272583a0. [DOI] [PubMed] [Google Scholar]
- Shepp D. H., Dandliker P. S., de Miranda P., Burnette T. C., Cederberg D. M., Kirk L. E., Meyers J. D. Activity of 9-[2-hydroxy-1-(hydroxymethyl)ethoxymethyl]guanine in the treatment of cytomegalovirus pneumonia. Ann Intern Med. 1985 Sep;103(3):368–373. doi: 10.7326/0003-4819-103-3-368. [DOI] [PubMed] [Google Scholar]
- Smee D. F., Boehme R., Chernow M., Binko B. P., Matthews T. R. Intracellular metabolism and enzymatic phosphorylation of 9-(1,3-dihydroxy-2-propoxymethyl)guanine and acyclovir in herpes simplex virus-infected and uninfected cells. Biochem Pharmacol. 1985 Apr 1;34(7):1049–1056. doi: 10.1016/0006-2952(85)90608-2. [DOI] [PubMed] [Google Scholar]
- Smee D. F., Martin J. C., Verheyden J. P., Matthews T. R. Anti-herpesvirus activity of the acyclic nucleoside 9-(1,3-dihydroxy-2-propoxymethyl)guanine. Antimicrob Agents Chemother. 1983 May;23(5):676–682. doi: 10.1128/aac.23.5.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. O., Galloway K. S., Kennell W. L., Ogilvie K. K., Radatus B. K. A new nucleoside analog, 9-[[2-hydroxy-1-(hydroxymethyl)ethoxyl]methyl]guanine, highly active in vitro against herpes simplex virus types 1 and 2. Antimicrob Agents Chemother. 1982 Jul;22(1):55–61. doi: 10.1128/aac.22.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]


