Abstract
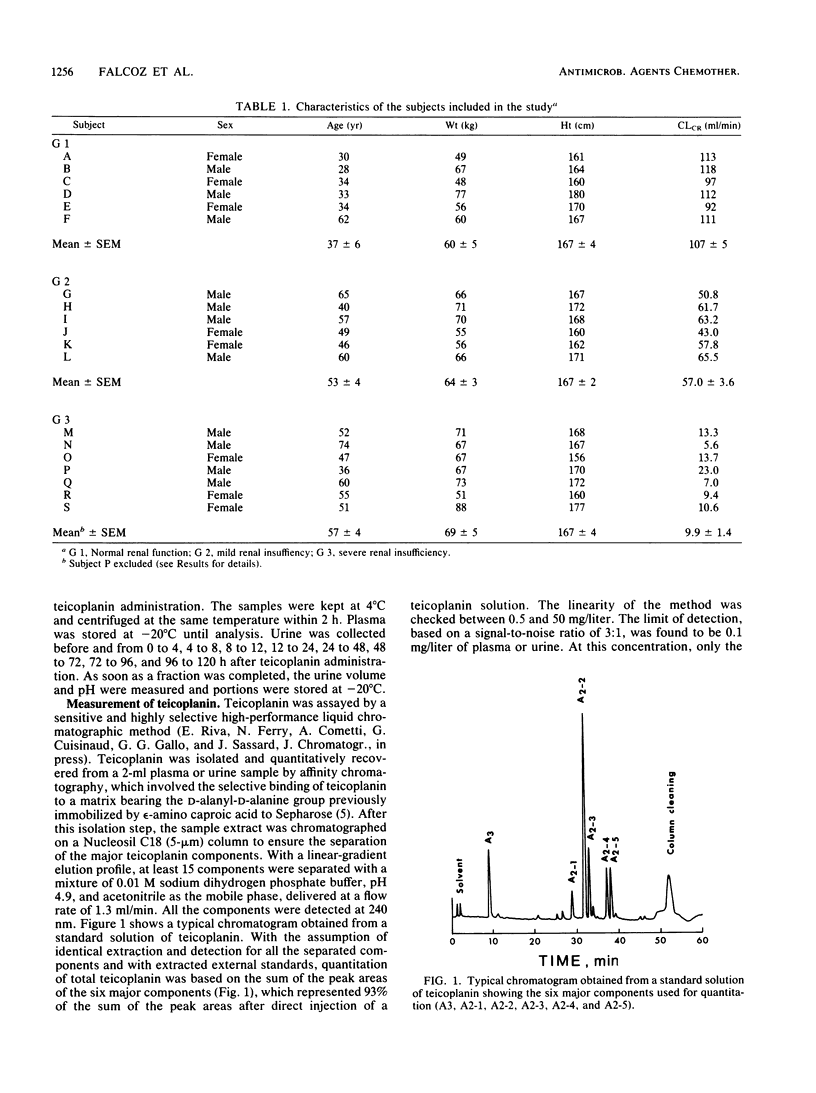
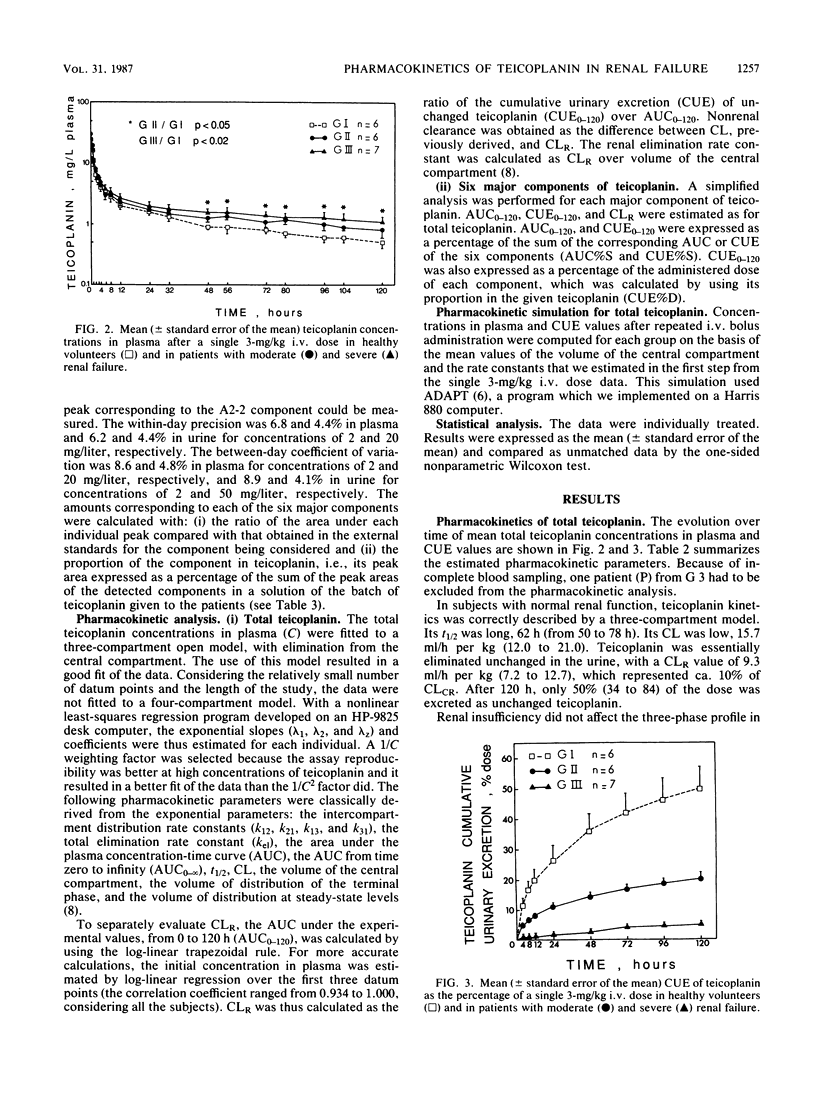
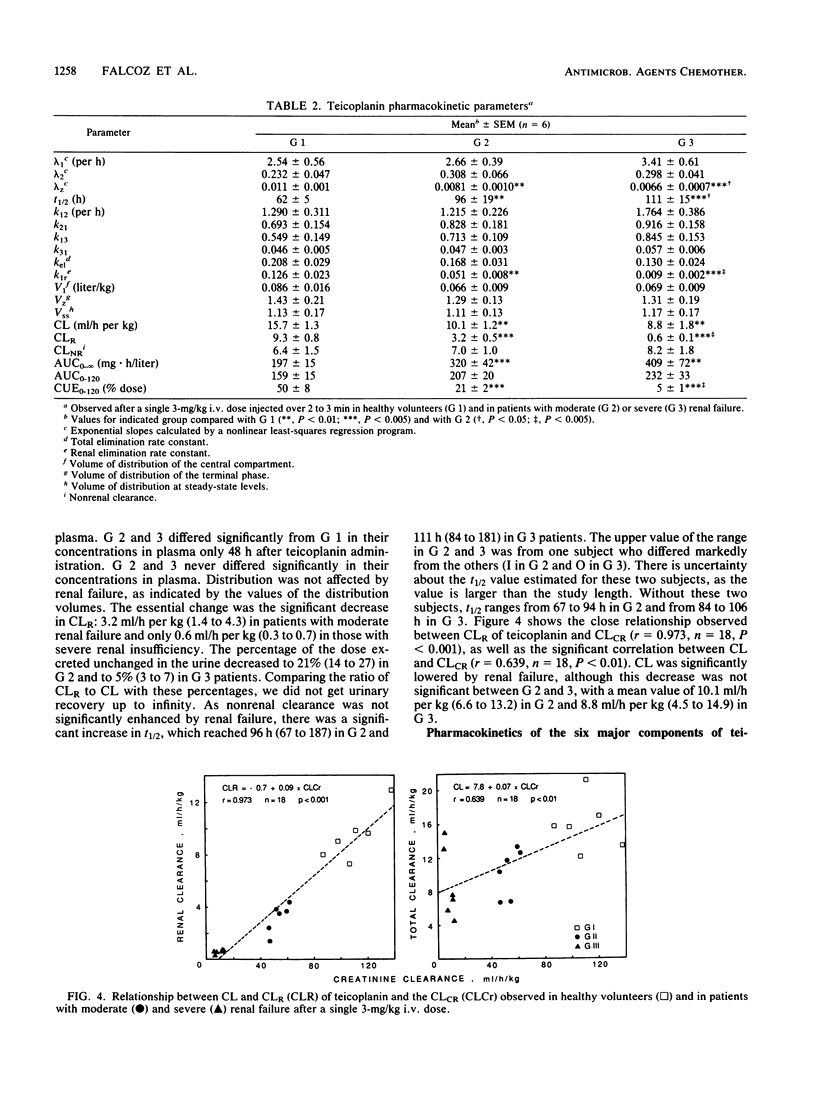
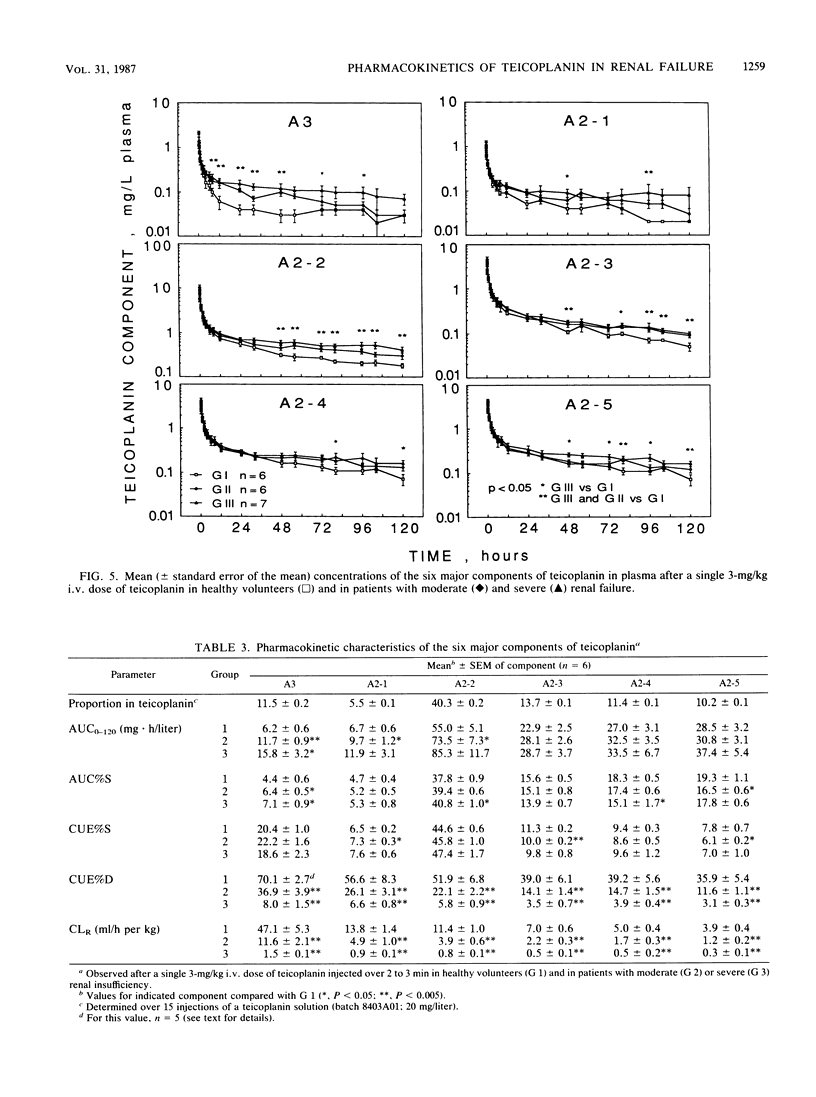
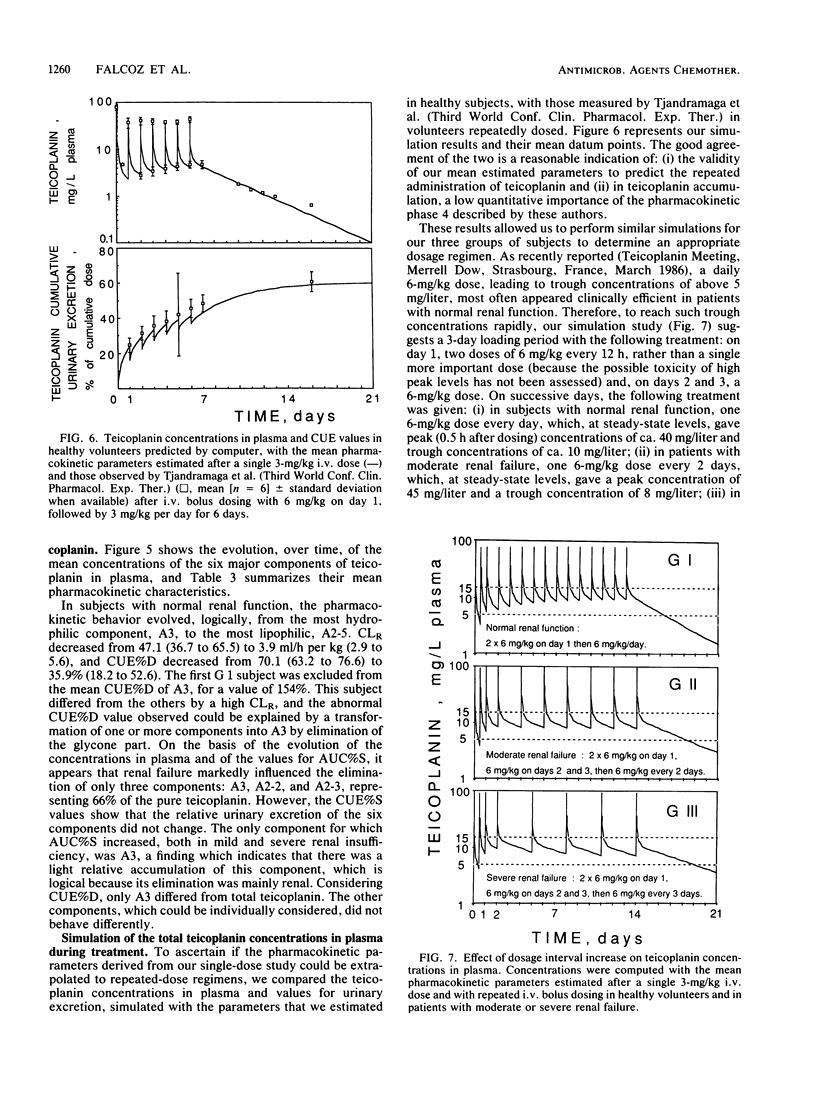
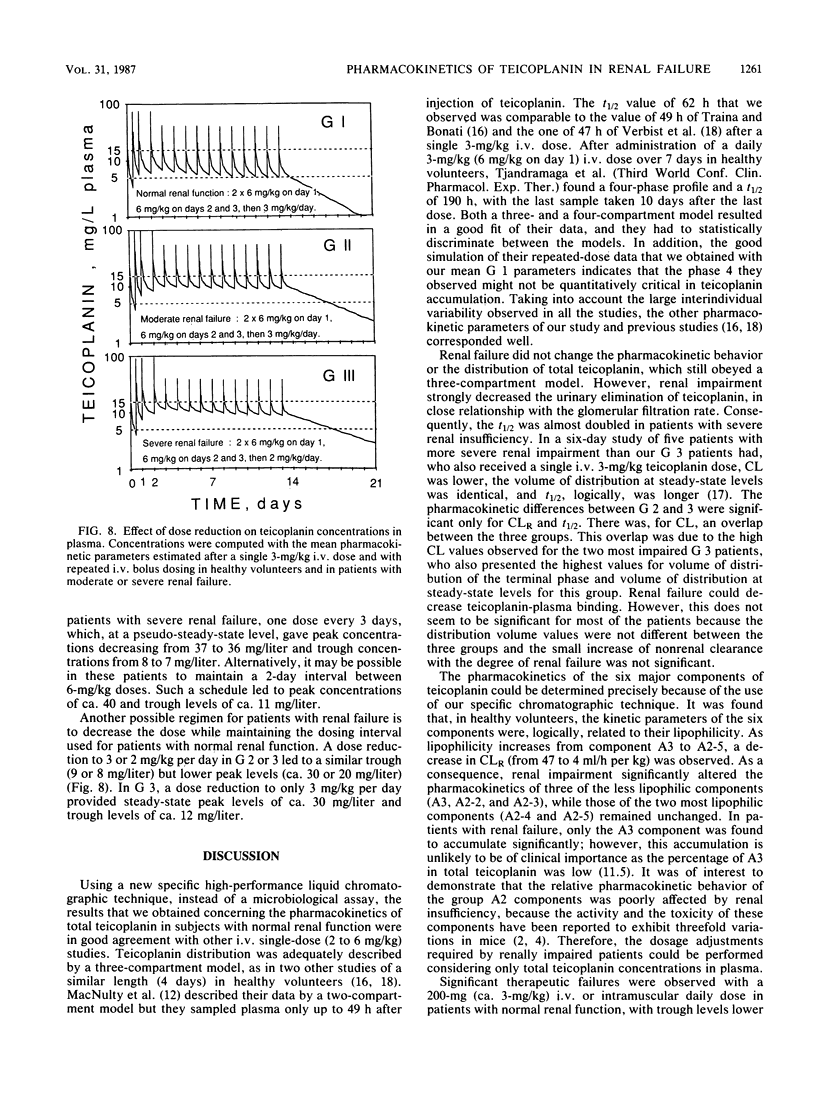
By using a highly specific chromatographic technique, the effect of renal failure on the pharmacokinetics of the six main components of teicoplanin, taken individually or as a whole, was assessed for over 120 h after administration of a 3-mg/kg intravenous dose to healthy volunteers (group 1, n = 6) and to noninfected patients with moderate (group 2, n = 6) or severe (group 3, n = 7) renal failure. In subjects with normal renal function, total teicoplanin was mainly excreted in urine and its concentrations in plasma could be adequately fitted to a three-compartment model. Renal failure did not affect the model or the distribution of teicoplanin but strongly decreased its renal clearance (9.3, 3.2, and 0.6 ml/h per kg, respectively, for the three groups of subjects), in close relationship with the creatinine clearance (r = 0.973, n = 18, P less than 0.001). The cumulative urinary excretion of unchanged total teicoplanin was decreased (50, 21, and 5% of the given dose for groups 1 to 3) and the terminal half-life was enhanced (62, 96, and 111 h for groups 1 to 3) by renal impairment. The relative behavior of the six major components was only slightly affected by renal failure. Consequently, the dosage regimen adjustment could be based on the total teicoplanin concentration, and simulations with the mean estimated pharmacokinetic parameters suggest that the 6-mg/kg daily dose, known to be effective in patients with normal renal function, could be given every 2 and 3 days in patients with moderate and severe renal insufficiency, respectively.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borghi A., Coronelli C., Faniuolo L., Allievi G., Pallanza R., Gallo G. G. Teichomycins, new antibiotics from Actinoplanes teichomyceticus nov. sp. IV. Separation and characterization of the components of teichomycin (teicoplanin). J Antibiot (Tokyo) 1984 Jun;37(6):615–620. doi: 10.7164/antibiotics.37.615. [DOI] [PubMed] [Google Scholar]
- Calain P., Krause K. H., Vaudaux P., Auckenthaler R., Lew D., Waldvogel F., Hirschel B. Early termination of a prospective, randomized trial comparing teicoplanin and flucloxacillin for treating severe staphylococcal infections. J Infect Dis. 1987 Feb;155(2):187–191. doi: 10.1093/infdis/155.2.187. [DOI] [PubMed] [Google Scholar]
- D'Argenio D. Z., Schumitzky A. A program package for simulation and parameter estimation in pharmacokinetic systems. Comput Programs Biomed. 1979 Mar;9(2):115–134. doi: 10.1016/0010-468x(79)90025-4. [DOI] [PubMed] [Google Scholar]
- Lagast H., Dodion P., Klastersky J. Comparison of pharmacokinetics and bactericidal activity of teicoplanin and vancomycin. J Antimicrob Chemother. 1986 Oct;18(4):513–520. doi: 10.1093/jac/18.4.513. [DOI] [PubMed] [Google Scholar]
- Matzke G. R., McGory R. W., Halstenson C. E., Keane W. F. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother. 1984 Apr;25(4):433–437. doi: 10.1128/aac.25.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty C. A., Garden G. M., Wise R., Andrews J. M. The pharmacokinetics and tissue penetration of teicoplanin. J Antimicrob Chemother. 1985 Dec;16(6):743–749. doi: 10.1093/jac/16.6.743. [DOI] [PubMed] [Google Scholar]
- Reed W. E., Jr, Sabatini S. The use of drugs in renal failure. Semin Nephrol. 1986 Sep;6(3):259–295. [PubMed] [Google Scholar]
- Somma S., Gastaldo L., Corti A. Teicoplanin, a new antibiotic from Actinoplanes teichomyceticus nov. sp. Antimicrob Agents Chemother. 1984 Dec;26(6):917–923. doi: 10.1128/aac.26.6.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traina G. L., Bonati M. Pharmacokinetics of teicoplanin in man after intravenous administration. J Pharmacokinet Biopharm. 1984 Apr;12(2):119–128. doi: 10.1007/BF01059273. [DOI] [PubMed] [Google Scholar]
- Traina G. L., Gentile M. G., Fellin G., Rosina R., Cavenaghi L., Buniva G., Bonati M. Pharmacokinetics of teicoplanin in patients on continuous ambulatory peritoneal dialysis. Eur J Clin Pharmacol. 1986;31(4):501–504. doi: 10.1007/BF00613532. [DOI] [PubMed] [Google Scholar]
- Verbist L., Tjandramaga B., Hendrickx B., Van Hecken A., Van Melle P., Verbesselt R., Verhaegen J., De Schepper P. J. In vitro activity and human pharmacokinetics of teicoplanin. Antimicrob Agents Chemother. 1984 Dec;26(6):881–886. doi: 10.1128/aac.26.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. H., Grüneberg R. N. Teicoplanin. J Antimicrob Chemother. 1984 Nov;14(5):441–445. [PubMed] [Google Scholar]


