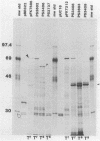
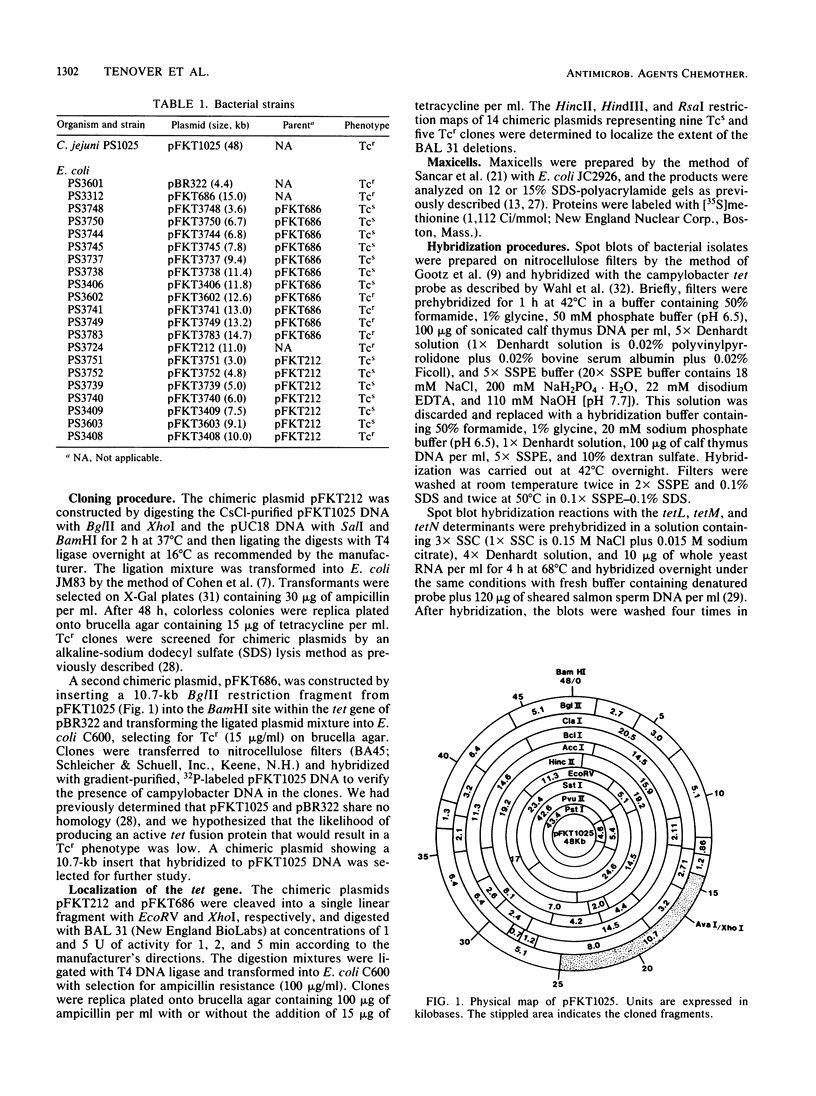
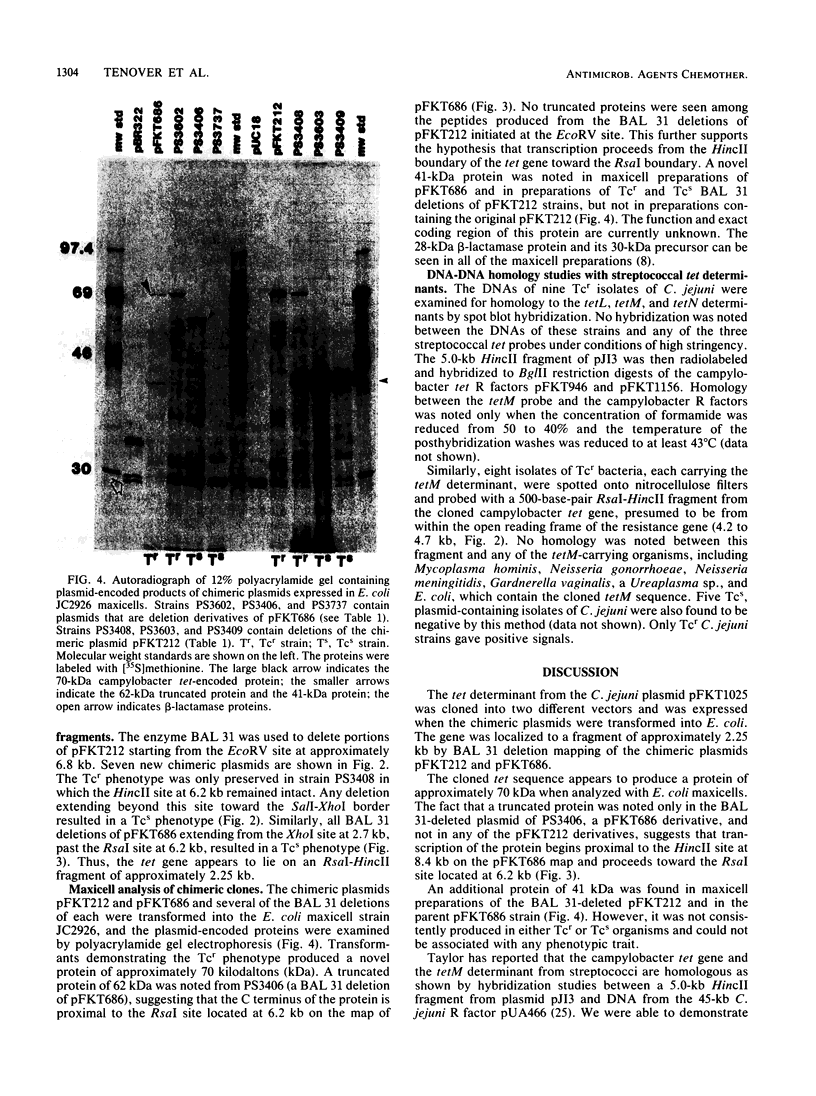
Abstract
The tetracycline resistance gene (tet) from the Campylobacter jejuni plasmid pFKT1025 was cloned into both pUC18 and pBR322 and was expressed when the chimeric plasmids were introduced into Escherichia coli. The location of the tet determinant on the chimeric plasmids was determined by BAL 31 deletion mapping within a 2.25-kilobase (kb) RsaI-HincII fragment. A protein of approximately 70 kilodaltons was consistently produced by E. coli maxicells harboring the cloned tet determinant. A 500-base-pair restriction fragment from within the 2.25-kb tet region was shown to hybridize only to DNA from tetracycline-resistant strains of C. jejuni and C. coli, but not to the DNA of organisms known to carry the streptococcal tetM determinant. No homology was noted between the DNA of 10 tetracycline-resistant isolates of campylobacter and the streptococcal tetL, tetM, or tetN determinants when tested under conditions of high stringency. However, homology was noted between a 5.0-kb HincII restriction fragment containing the tetM determinant and two C. jejuni tet R factors under conditions of reduced stringency.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Prody C., Kustu S. Simple, rapid, and quantitative release of periplasmic proteins by chloroform. J Bacteriol. 1984 Dec;160(3):1181–1183. doi: 10.1128/jb.160.3.1181-1183.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Reller L. B. Campylobacter enteritis. N Engl J Med. 1981 Dec 10;305(24):1444–1452. doi: 10.1056/NEJM198112103052404. [DOI] [PubMed] [Google Scholar]
- Buck G. E., Kelly M. T. Susceptibility testing of Campylobacter fetus subsp. jejuni, using broth microdilution panels. Antimicrob Agents Chemother. 1982 Feb;21(2):274–277. doi: 10.1128/aac.21.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett V., Inamine J., Rajagopalan S. Heterogeneity of tetracycline resistance determinants in Streptococcus. J Bacteriol. 1982 Mar;149(3):995–1004. doi: 10.1128/jb.149.3.995-1004.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett V. Streptococcal tetracycline resistance mediated at the level of protein synthesis. J Bacteriol. 1986 Feb;165(2):564–569. doi: 10.1128/jb.165.2.564-569.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan G., Saul M., Twigg A., Gill R., Sherratt D. Polypeptides expressed in Escherichia coli K-12 minicells by transposition elements Tn1 and Tn3. J Bacteriol. 1979 Apr;138(1):48–54. doi: 10.1128/jb.138.1.48-54.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootz T. D., Tenover F. C., Young S. A., Gordon K. P., Plorde J. J. Comparison of three DNA hybridization methods for detection of the aminoglycoside 2"-O-adenylyltransferase gene in clinical bacterial isolates. Antimicrob Agents Chemother. 1985 Jul;28(1):69–73. doi: 10.1128/aac.28.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris N. V., Thompson D., Martin D. C., Nolan C. M. A survey of Campylobacter and other bacterial contaminants of pre-market chicken and retail poultry and meats, King County, Washington. Am J Public Health. 1986 Apr;76(4):401–406. doi: 10.2105/ajph.76.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris N. V., Weiss N. S., Nolan C. M. The role of poultry and meats in the etiology of Campylobacter jejuni/coli enteritis. Am J Public Health. 1986 Apr;76(4):407–411. doi: 10.2105/ajph.76.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmali M. A., De Grandis S., Fleming P. C. Antimicrobial susceptibility of Campylobacter jejuni with special reference to resistance patterns of Canadian isolates. Antimicrob Agents Chemother. 1981 Apr;19(4):593–597. doi: 10.1128/aac.19.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martin P., Trieu-Cuot P., Courvalin P. Nucleotide sequence of the tetM tetracycline resistance determinant of the streptococcal conjugative shuttle transposon Tn1545. Nucleic Acids Res. 1986 Sep 11;14(17):7047–7058. doi: 10.1093/nar/14.17.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel J., Rogol M., Dickman D. Susceptibility of clinical isolates of Campylobacter jejuni to sixteen antimicrobial agents. Antimicrob Agents Chemother. 1983 May;23(5):796–797. doi: 10.1128/aac.23.5.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Johnson S. R., Biddle J. W., Roberts M. C. High-level tetracycline resistance in Neisseria gonorrhoeae is result of acquisition of streptococcal tetM determinant. Antimicrob Agents Chemother. 1986 Nov;30(5):664–670. doi: 10.1128/aac.30.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. C., Kenny G. E. Dissemination of the tetM tetracycline resistance determinant to Ureaplasma urealyticum. Antimicrob Agents Chemother. 1986 Feb;29(2):350–352. doi: 10.1128/aac.29.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. C., Koutsky L. A., Holmes K. K., LeBlanc D. J., Kenny G. E. Tetracycline-resistant Mycoplasma hominis strains contain streptococcal tetM sequences. Antimicrob Agents Chemother. 1985 Jul;28(1):141–143. doi: 10.1128/aac.28.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Strom M. S., Lory S. Cloning and expression of the pilin gene of Pseudomonas aeruginosa PAK in Escherichia coli. J Bacteriol. 1986 Feb;165(2):367–372. doi: 10.1128/jb.165.2.367-372.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedhem A., Kaijser B., Sjögren E. Antimicrobial susceptibility of Campylobacter jejuni isolated from humans with diarrhoea and from healthy chickens. J Antimicrob Chemother. 1981 Mar;7(3):301–305. doi: 10.1093/jac/7.3.301. [DOI] [PubMed] [Google Scholar]
- Taylor D. E., Garner R. S., Allan B. J. Characterization of tetracycline resistance plasmids from Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother. 1983 Dec;24(6):930–935. doi: 10.1128/aac.24.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E. Plasmid-mediated tetracycline resistance in Campylobacter jejuni: expression in Escherichia coli and identification of homology with streptococcal class M determinant. J Bacteriol. 1986 Mar;165(3):1037–1039. doi: 10.1128/jb.165.3.1037-1039.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F. C., Gootz T. D., Gordon K. P., Tompkins L. S., Young S. A., Plorde J. J. Development of a DNA probe for the structural gene of the 2"-O-adenyltransferase aminoglycoside-modifying enzyme. J Infect Dis. 1984 Nov;150(5):678–687. doi: 10.1093/infdis/150.5.678. [DOI] [PubMed] [Google Scholar]
- Tenover F. C., Williams S., Gordon K. P., Nolan C., Plorde J. J. Survey of plasmids and resistance factors in Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother. 1985 Jan;27(1):37–41. doi: 10.1128/aac.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer R. E. An improved method for detecting foreign DNA in plasmids of Escherichia coli. Anal Biochem. 1979 Sep 15;98(1):60–63. doi: 10.1016/0003-2697(79)90705-x. [DOI] [PubMed] [Google Scholar]
- Vanhoof R., Gordts B., Dierickx R., Coignau H., Butzler J. P. Bacteriostatic and bactericidal activities of 24 antimicrobial agents against Campylobacter fetus subsp. jejuni. Antimicrob Agents Chemother. 1980 Jul;18(1):118–121. doi: 10.1128/aac.18.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]