Abstract
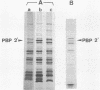
Methicillin- and cephem-resistant Staphylococcus aureus (137 strains) for which the cefazolin MICs are at least 25 micrograms/ml could be classified into low-resistance (83% of strains) and high-resistance (the remaining 17%) groups by the MIC of flomoxef (6315-S), a 1-oxacephalosporin. The MICs were less than 6.3 micrograms/ml and more than 12.5 micrograms/ml in the low- and high-resistance groups, respectively. All strains produced penicillin-binding protein 2' (PBP 2'), which has been associated with methicillin resistance and which has very low affinity for beta-lactam antibiotics. Production of PBP 2' was regulated differently in low- and high-resistance strains. With penicillinase-producing strains of the low-resistance group, cefazolin, cefamandole, and cefmetazole induced PBP 2' production about 5-fold, while flomoxef induced production 2.4-fold or less. In contrast, penicillinase-negative variants of low-resistance strains produced PBP 2' constitutively in large amounts and induction did not occur. With high-resistance strains, flomoxef induced PBP 2' to an extent similar to that of cefazolin in both penicillinase-producing and -negative strains, except for one strain in which the induction did not occur. The amount of PBP 2' induced by beta-lactam antibiotics in penicillinase-producing strains of the low-resistance group correlated well with resistance to each antibiotic. Large amounts of PBP 2' in penicillinase-negative variants of the low-resistance group did not raise the MICs of beta-lactam compounds, although these strains were more resistant when challenged with flomoxef for 2 h. Different regulation of PBP 2' production was demonstrated in the high- and low-resistance groups, and factor(s) other than PBP 2' were suggested to be involved in the methicillin resistance of high-resistance strains.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARBER M. Methicillin-resistant staphylococci. J Clin Pathol. 1961 Jul;14:385–393. doi: 10.1136/jcp.14.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck W. D., Berger-Bächi B., Kayser F. H. Additional DNA in methicillin-resistant Staphylococcus aureus and molecular cloning of mec-specific DNA. J Bacteriol. 1986 Feb;165(2):373–378. doi: 10.1128/jb.165.2.373-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. F., Reynolds P. E. Intrinsic resistance to beta-lactam antibiotics in Staphylococcus aureus. FEBS Lett. 1980 Dec 29;122(2):275–278. doi: 10.1016/0014-5793(80)80455-8. [DOI] [PubMed] [Google Scholar]
- Chambers H. F., Hartman B. J., Tomasz A. Increased amounts of a novel penicillin-binding protein in a strain of methicillin-resistant Staphylococcus aureus exposed to nafcillin. J Clin Invest. 1985 Jul;76(1):325–331. doi: 10.1172/JCI111965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Sweeney H. M. Effect of the prophage and penicillinase plasmid of the recipient strain upon the transduction and the stability of methicillin resistance in Staphylococcus aureus. J Bacteriol. 1973 Nov;116(2):803–811. doi: 10.1128/jb.116.2.803-811.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Smith S. A., Bonner D. P. Penicillin-binding proteins in a Staphylococcus aureus strain resistant to specific beta-lactam antibiotics. Antimicrob Agents Chemother. 1982 Jul;22(1):172–175. doi: 10.1128/aac.22.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Expression of methicillin resistance in heterogeneous strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Jan;29(1):85–92. doi: 10.1128/aac.29.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984 May;158(2):513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman B., Tomasz A. Altered penicillin-binding proteins in methicillin-resistant strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1981 May;19(5):726–735. doi: 10.1128/aac.19.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono M., Sasatsu M., O'Hara K., Shiomi Y., Hayasaka T. Mechanism of resistance to some cephalosporins in Staphylococcus aureus. Antimicrob Agents Chemother. 1983 Jun;23(6):938–940. doi: 10.1128/aac.23.6.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAY J. W., HOUGHTON R. H., PERRET C. J. THE EFFECT OF GROWTH AT ELEVATED TEMPERATURES ON SOME HERITABLE PROPERTIES OF STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1964 Nov;37:157–169. doi: 10.1099/00221287-37-2-157. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regamey C., Libke R. D., Engelking E. R., Clarke J. T., Kirby M. M. Inactivation of cefazolin, cephaloridine, and cephalothin by methicillin-sensitive and methicillin-resistant strains of Staphylococcus aureus. J Infect Dis. 1975 Mar;131(3):291–294. doi: 10.1093/infdis/131.3.291. [DOI] [PubMed] [Google Scholar]
- Reynolds P. E., Brown D. F. Penicillin-binding proteins of beta-lactam-resistant strains of Staphylococcus aureus. Effect of growth conditions. FEBS Lett. 1985 Nov 11;192(1):28–32. doi: 10.1016/0014-5793(85)80036-3. [DOI] [PubMed] [Google Scholar]
- Rossi L., Tonin E., Cheng Y. R., Fontana R. Regulation of penicillin-binding protein activity: description of a methicillin-inducible penicillin-binding protein in Staphylococcus aureus. Antimicrob Agents Chemother. 1985 May;27(5):828–831. doi: 10.1128/aac.27.5.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabath L. D. Mechanisms of resistance to beta-lactam antibiotics in strains of Staphylococcus aureus. Ann Intern Med. 1982 Sep;97(3):339–344. doi: 10.7326/0003-4819-97-3-339. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Stewart G. C., Rosenblum E. D. Transduction of methicillin resistance in Staphylococcus aureus: recipient effectiveness and beta-lactamase production. Antimicrob Agents Chemother. 1980 Sep;18(3):424–432. doi: 10.1128/aac.18.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T., Satoh H., Narisada M., Hamashima Y., Yoshida T. Synthesis and antibacterial activity of 6315-S, a new member of the oxacephem antibiotic. J Antibiot (Tokyo) 1985 Apr;38(4):466–476. doi: 10.7164/antibiotics.38.466. [DOI] [PubMed] [Google Scholar]
- Ubukata K., Yamashita N., Konno M. Occurrence of a beta-lactam-inducible penicillin-binding protein in methicillin-resistant staphylococci. Antimicrob Agents Chemother. 1985 May;27(5):851–857. doi: 10.1128/aac.27.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsui Y., Yokota T. Role of an altered penicillin-binding protein in methicillin- and cephem-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1985 Sep;28(3):397–403. doi: 10.1128/aac.28.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]