Abstract
Complex biological processes require coordinated function of many genes. One evolutionary solution to the problem of coordinately expressing functionally related genes in bacteria and nematodes is organization of genes in operons. Surprisingly, eukaryotic operons are considered rare outside the nematode lineage. In Drosophila melanogaster, we found lounge lizard (llz), which encodes a degenerin/ENaC cation channel, cotranscribed with CheB42a, a nonhomologous gene of unknown function residing <100 bp upstream. These two genes were transcribed from a single promoter as one primary transcript and were processed posttranscriptionally to generate individual mRNAs. The mechanism did not involve alternative splicing, and it differed from the trans splicing used in nematode operons. Both genes were expressed in the same tissues, and previous work suggested that both may be involved in courtship behavior. A bioinformatic approach identified numerous additional loci as potential Drosophila operons. These data reveal eukaryotic operon-like transcription of functionally related genes in Drosophila. The results also suggest that operon-based transcription may be more common in eukaryotes than previously appreciated.
Keywords: degenerin/epithelial Na+ channel, fruit fly, ion channels, lounge lizard, CheB42a
Degenerin/epithelial Na+ channel (DEG/ENaC) proteins form non-voltage-gated cation channels. Their functions vary widely and include mechanosensation, nociception (sensing acidic stimuli), salt sensing, and detecting the peptidergic neurotransmitter FMRFamide (1–4). During our investigation of Drosophila melanogaster DEG/ENaC channels, we discovered a genomic locus containing a DEG/ENaC gene, which we named lounge lizard (llz, CG33349), and a nonhomologous gene of unknown function (CheB42a) (5) that appeared to be transcribed in the same direction with a predicted intergenic distance of <100 bp. The close proximity of these two genes and the lack of obvious promoter sequences in front of the downstream gene suggested that they might be cotranscribed from a single upstream promoter. This organization would resemble that of the nematode operon, an elegant evolutionary solution for coexpressing genes that are part of the same biochemical pathway or physiological process (6, 7).
Although abundant in nematodes, operons are thought to be rare in other eukaryotes. This is despite eukaryote's complicated cellular physiology and the intricacy of their genomes, which might predict that operon-like transcription would solve some of the problem of coordinately regulating genes (8). With this background, we hypothesized that the CheB42a/llz locus might be transcribed as a eukaryotic operon, and, hence, the two genes might be functionally related.
Results
Organization of the CheB42a/llz Locus is Conserved Across Drosophila Species.
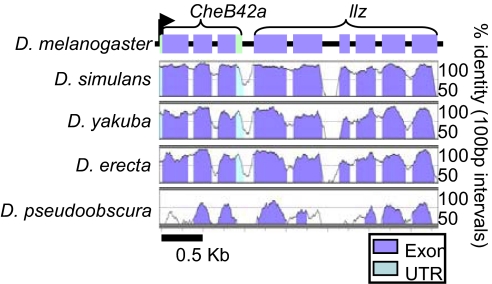
In the D. melanogaster genome, llz is located immediately downstream of a previously cloned gene, CheB42a (5). Transcription of the two genes appeared to be in the same direction, and both have multiple exons (Fig. 1). The two gene products belong to different protein families. Genomes of other Drosophila species contain the same two genes in a similar organization (Fig. 1), raising the possibility that the arrangement imparts some function. Examination of the genomic sequence revealed that the 3′ end of CheB42a contains a poly(A) addition sequence, predicting that CheB42a should generate an independent mRNA. However, we detected no obvious promoter elements in the 97-bp intergenic sequence between CheB42a and llz, suggesting that if it is expressed, then the llz gene may be controlled by upstream sequences.
Fig. 1.
The CheB42a/llz operon-like locus is conserved across Drosophila species. VISTA plots of the CheB42a/llz locus in various Drosophila species with D. melanogaster as the reference genome. Order of species represents phylogenetic distance from D. melanogaster with closest species (D. simulans) at the top. Curves represent % conservation in a continuous 100-bp interval.
These genomic features suggested that the CheB42a/llz locus might be transcribed as an operon. In nematode operons, one promoter/enhancer generates a primary transcript (or pre-mRNA) for two or more genes (6, 9). Caenorhabditis elegans process this primary transcript into individual mRNAs for each gene and attach a specific spliced leader sequence (SL2) to the 5′ ends of mRNAs from the downstream genes. Nematode operons often encode genes contributing to a common biological process, just as bacterial operons usually code for proteins in a shared pathway (10).
CheB42a and llz Are Cotranscribed from a Single Promoter.
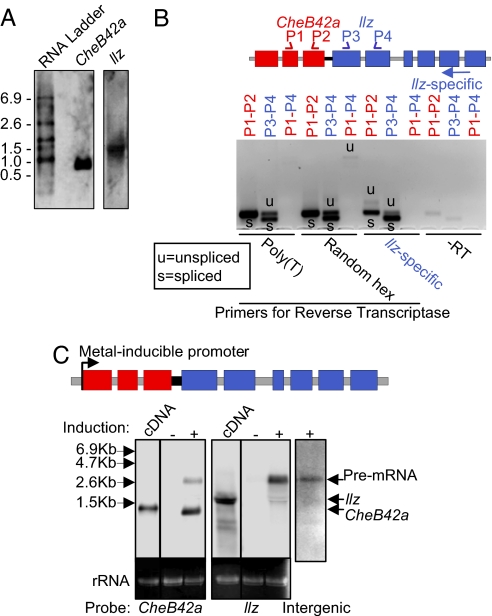
To test the hypothesis that the CheB42a/llz locus undergoes operon-like transcription, we first asked whether both genes are transcribed. Northern blots of whole flies revealed independent transcripts for both CheB42a and llz (Fig. 2a).
Fig. 2.
The CheB42a/llz locus is transcribed as a single pre-mRNA and is processed posttranscriptionally. (A) Northern blot analysis of CheB42a and llz expressed in flies. Same membrane was probed for both transcripts. Left lane shows an RNA-size marker. (B) RT-PCR of total RNA extracted from male appendages. RT step was performed with poly(T), random hexamers, or llz-specific primers. “−RT” indicates reaction without reverse transcriptase. P1–P4 represents primers used in the PCR step. Primers P1 and P2 are CheB42a-specific and primers P3 and P4 are llz-specific. Multiple clones from all PCR products were sequenced to verify their identity. Some PCR products from P1–P4 primers had some of the introns variably spliced, further indicating that they were not due to genomic contamination. (C) Northern blot of CheB42a and llz transcribed from an exogenous inducible upstream promoter. A genomic fragment containing all exons and introns of CheB42a and llz was subcloned into a vector containing a metal-inducible promoter and transfected into Drosophila S2 cells. cDNAs of both genes were transfected as controls. RNA produced from the CheB42a cDNA is slightly larger than the spliced product from the genomic construct due to the presence of V5 and 6× His tags sequences in the CheB42a cDNA. The llz-specific signal showed two bands when expressed from both the cDNA and the genomic construct, which may indicate alternative lengths of poly(A) tails. Pre-mRNA also was identified with a probe to the intergenic region (right lane). Failure to detect the pre-mRNA on Northern blot analysis (A) is likely because of its low abundance, as has been observed in C. elegans where operon processing is very efficient (6).
We then used RT-PCR to determine whether the locus also produced a primary transcript containing both genes. Primers for the reverse transcription (RT) reaction included as follows: (i) poly(T), which allows reverse transcription of polyadenylated mRNA; (ii) random hexamers, which reverse transcribes all RNAs; and (iii) an llz-specific primer (Fig. 2B). PCR primers spanned the last intron of CheB42a, the first intron of llz, or both plus the intergenic region. With the poly(T) and random hexamer primers, PCR generated the predicted spliced products from CheB42a and llz (Fig. 2B), and products from the poly(T) reaction suggested polyadenylation of both CheB42a and llz mRNAs. We also found PCR products extending from the CheB42a to the llz locus; cloning and sequencing of those products showed that they retained the intergenic region, but some had introns variably spliced out. These data suggested that a single pre-mRNA contained both genes. Importantly, when we primed the RT reaction with an llz-specific primer, PCR generated spliced CheB42a products and unspliced CheB42a and CheB42a-llz products (Fig. 2B). Finding a primary transcript spanning both genes suggested operon-like transcription.
If the CheB42a/llz locus functions as an operon, we reasoned that an exogenous promoter inserted upstream of the locus also should generate a primary transcript containing sequences from CheB42a and llz plus individual transcripts for each gene. To test this prediction, we cloned a complete genomic fragment of the CheB42a/llz locus without its endogenous promoter (i.e., upstream of CheB42a) into a metal-inducible expression vector and transfected Drosophila S2 cells (Fig. 2C). Without induction, we detected neither transcript. This result suggests that the intergenic region and CheB42a introns do not contain promoter activity that drives llz expression independent of CheB42a. After inducing transcription with copper sulfate, Northern blots probed for CheB42a and llz revealed an RNA species encoding both genes plus mature transcripts for each individual gene. The identity of the primary transcript also was verified by an intergenic probe (Fig. 2C). The ratio of mature mRNA to primary transcript was higher for CheB42a than for llz; this result suggests that processing of the downstream gene was less efficient than the upstream gene, a finding consistent with tissue expression levels (Fig. 2A) and operon processing in C. elegans (11).
As an additional test for an unrecognized promoter that might drive llz transcription, we cloned a genomic fragment extending from the start of the first CheB42a exon through the intergenic sequence and used it to drive a GFP reporter gene, using the UAS-GAL4 system. In flies transgenic for this construct, we failed to detect GFP expression (data not shown), consistent with the lack of a promoter downstream of the CheB42a transcriptional start site.
Together, these results indicate that an upstream promoter generated a single pre-mRNA that contained sequences for both genes. The pre-mRNA then was processed to two mature transcripts, one for CheB42a and one for llz. Thus, this locus exhibits processing that shares some features with C. elegans operons (6). In contrast, the CheB42a/llz locus behaves differently from the Drosophila stoned locus, in which a single mature (spliced) transcript encodes two different proteins (i.e., a dicistronic mRNA) (12). We excluded this alternative because Northern blots from fly tissues revealed two independent transcripts (Fig. 2A). In addition, the RT-PCR results (Fig. 2B) and the CheB42a 3′ RACE and llz 5′ RACE results (see below) failed to identify fully spliced transcripts containing both CheB42a and llz sequences.
Analysis of CheB42a and llz Transcripts.
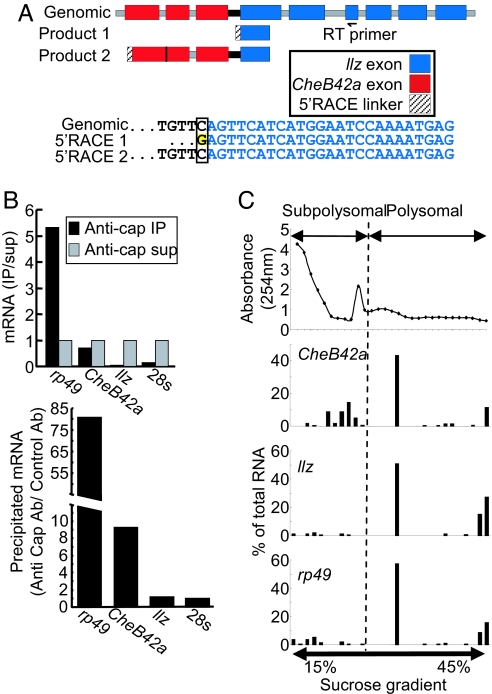
Transcripts encoding two unrelated proteins also could arise from one primary transcript by alternative splicing; an example is the Drosophila cholinergic locus (13). This mechanism predicts that the two mature mRNAs will share some 5′ UTR sequences. To test this possibility, we used 5′ RACE to define the transcriptional start sites of CheB42a and llz. We found that mature (all introns spliced out) llz transcripts shared no sequence with CheB42a transcripts (Fig. 3A). Lack of a shared 5′ UTR excludes alternative splicing as the mechanism for individual transcript processing in at this locus.
Fig. 3.
The downstream llz transcript does not contain traditional 5′ cap llz 5′ RACE analysis. (A) Schematic of genomic (Upper) and 5′ RACE (Lower) structure. (A Lower) Genomic sequence and sequence of 5′ end of typical llz products with the extra nontemplate G highlighted in yellow (product 1). Product 2 indicates sequence in same region of a partially spliced product that extended through CheB42a. (B) Immuoprecipitation of total RNA with an anti-cap antibody. Total RNA from male appendages was precipitated with an anti-cap or a control antibody. Relative amounts of mRNAs were detected with real-time quantitative RT-PCR with a gene-specific probe and primers. The ribosomal gene rp49 served as a positive control for a capped mRNA, and the 28s ribosomal RNA served as an uncapped negative control. (B Upper) Relative amount of precipitated RNA compared with the amount of RNA remaining in the supernatant; low levels of precipitated llz were not simply the result of low levels of llz mRNA. (C) Analysis of polysomal RNA. S2 cells were transfected with the same genomic construct as in Fig. 2C. Cytosolic suspension was loaded on a 15–45% sucrose gradient, and total RNA was isolated from each fraction. (C Top) Absorbance at 254 nm for each fraction, which was used as a guide to indicate subpolysomal and polysomal fractions. (Middle Top, Middle Bottom, and Bottom) Real-time quantitative RT-PCR (shown as percentage of total mRNA) results for CheB42a, llz, and rp49 (as a capped control), respectively.
In C. elegans operons, primary transcripts are processed to individual mRNAs with trans splicing of a specific spliced-leader sequence (SL2) to the 5′ end of mRNA from the downstream gene (6). Our 5′ RACE analysis revealed no splice-leader sequence attached to the llz transcript, suggesting that a different mechanism was responsible for 5′ end formation of the llz gene. In attempting 5′ RACE, on multiple occasions, we first selected capped mRNAs, but although we readily detected CheB42a products, we did not generate llz-specific products. However, when we omitted selection for capped mRNA, we recovered products from both genes. These results suggested that llz transcripts might not bear a traditional 5′ cap. To further test this hypothesis, we used an anti-cap antibody to immunoprecipitate capped RNAs from fly tissues. The anti-cap antibody precipitated CheB42a, but not llz transcripts, which remained in the supernatant (Fig. 3B). These results suggest that llz transcripts do not bear a conventional cap.
Interestingly, at the 5′ end of llz 5′ RACE products, we found a guanosine that was not encoded by genomic DNA (Fig. 3A). We did not find a guanosine in partial cDNAs, and genomic DNA sequencing of the llz 5′ UTR region showed this did not arise from a DNA polymorphism. These data indicate that the additional guanosine was added posttranscriptionally during 5′ end formation of llz and suggest it may be an important element in processing the llz transcript. We considered that the guanosine might have been added in a self-splicing reaction analogous to that which occurs in group I introns, although in that case, the quanosine is added to intron- rather than exon-encoded sequence (14). Nevertheless, we tested for group I intron-like activity but found no processing of the locus under in vitro conditions used to study group I introns (data not shown). Although we do not know the mechanism involved, these results suggest that the guanosine might be added posttranscriptionally, possibly as part of the 5′ end formation of llz.
We asked whether llz produces functional mRNA by testing for spliced llz transcripts in polyribosomes, the sites of protein synthesis (15). We expressed the CheB42a/llz genomic locus in Drosophila S2 cells (as described above for Fig. 3C) and collected sucrose gradient fractions to separate subpopulations of ribosome-bound cellular mRNAs. We found llz transcripts in the same fractions as CheB42a and rp49 (a ribosomal protein used as a positive control). These data indicate that although llz may not contain a traditional cap, it attracted the translational machinery and, hence, is very likely a functional mRNA. Of note, cap-independent translation has been reported for eukaryotic mRNAs (16).
Other D. melanogaster Genes also May Be Transcribed as Operons.
Operons are common in C. elegans (7). This suggested that the CheB42a/llz locus might not be a unique case in Drosophila, but rather represent a general pattern of chromosomal organization. To evaluate this possibility, we examined the D. melanogaster genome. Using an arbitrary and conservative maximum length of 700 bp for the intergenic region, we identified 2,468 pairs of genes transcribed in the same direction. Of these, 1,378 gene pairs had no predicted promoter sequences in the intergenic region [supporting information (SI) Table 1]. Requiring a stop codon in the upstream gene and polyadenylation signal sequences in the 3′ UTR of both genes further reduced the number to 409 gene pairs. Of these, 397 were pairs of nonhomologous genes. These features are hallmarks of operons in the C. elegans genome (7) and suggest that the Drosophila genome contains numerous loci that might be transcribed in a similar fashion.
To test whether any of these gene pairs are possible operons, we chose two candidate loci containing nonhomologous pairs of genes: Tektin-C/CG10542 and Cdk5/flotillin. Both loci were conserved across Drosophila species (SI Fig. 6 A and B). We then used an RT-PCR analysis similar to what we reported in Fig. 2B for the CheB42a/llz locus. PCR primers crossed introns in each gene (SI Fig. 6 A and B). When we used a CG10542 (the downstream gene) primer for the RT reaction, we found Tektin-C (the upstream gene) PCR products that had an intron spliced out (SI Fig. 6C and confirmed by DNA sequencing). This result indicates that the locus generated a single pre-mRNA that contained both genes. The functions of Tektin-C and CG10542 encoded proteins are unknown, but both may be involved with cytoskeleton organization (www.flybase.org). We obtained similar RT-PCR results from the Cdk5/flotillin locus; RT-PCR products representing spliced transcripts were derived from both genes (SI Fig. 6D). flotillin is an integral membrane protein and Cdk5 (cyclin-dependent kinase 5) is a soluble protein (17, 18). Although they are not known to participate in a common biochemical pathway, they both contribute to neuronal signal transduction, and both have been implicated in the pathogenesis of Alzheimer's disease in humans (19, 20). These data suggest that these two additional loci also may show an operon-like organization.
Because llz transcripts begin with a guanosine not encoded by genomic DNA, we searched for this feature in ESTs from the downstream genes present in our candidate list. We found an EST for 392 of the 409 downstream genes. In 26 cases (6.6%), we discovered a leading guanosine not encoded by the genomic template. Other noncoded nucleotides were rare (1 T, 3 A, and 2 C) in these 392 ESTs. Moreover, two random EST sets had a nontemplate G in 4 of 392 (1.0%) and 6 of 392 (1.5%) ESTs (P < 0.0005 for both by Fisher's exact test). These data are consistent with the hypothesis that the Drosophila genome contains additional operon-like loci and that a specialized, although as-yet-unknown, mechanism may be involved in their processing.
Production of Separate CheB42a and llz Transcripts Requires Specific Sequences.
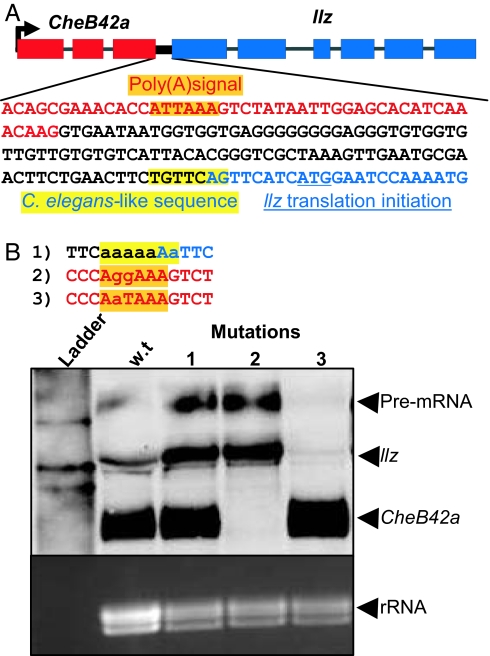
Processing of C. elegans operons requires specific sequence elements in the intergenic region and the 3′ UTR of the upstream gene (11, 21, 22). Therefore, we mutated these regions to learn whether they also influence production of individual CheB42a and llz transcripts; we used the construct shown in Fig. 2C. The 3′ end of the CheB42a-llz intergenic region (Fig. 4A) is conserved across Drosophila species (data not shown). Although it is not conserved in the 409 gene pairs, it is similar to a critical 3′ end sequence of some C. elegans operons (22). Mutating this sequence (mutation 1; Fig. 4B) increased the amount of pre-mRNA and mature llz transcripts relative to the wild-type construct. These data suggest that intergenic sequences contribute to processing of the locus.
Fig. 4.
Maturation of CheB42a and llz mRNA requires specific sequences. (A) Intergenic region of the CheB42a/llz locus. Red letters represent 3′ end of the last exon in CheB42a. Orange box indicates predicted polyadenylation signal for CheB42a. Blue letters indicate start of the first exon of llz. Blue underline indicates translation start site for llz. Yellow box represents conserved sequence found at 3′ end of intercistronic region in some C. elegans operons. Boundaries of the CheB42a and llz transcripts and the intergenic region were determined by 3′and 5′ RACE. (B) Effect of mutagenesis on processing of the CheB42a/llz locus. Sequences represent the mutations studied. Colored boxes correspond to colored boxes in A. Mutations are in lowercase letters. Northern blot was incubated with probes for CheB42a and llz simultaneously.
Previous work in C. elegans suggested that an interplay between the poly(A) signal of the upstream gene and downstream intergenic sequences is important for operon processing (22). We found that disrupting the CheB42a poly(A) signal eliminated mature CheB42a transcripts while increasing pre-mRNA and llz transcripts (mutant 2; Fig. 4B). Formation of llz from this mutant suggests that llz and CheB42a may mature independently of each other. The loss of mature CheB42a transcripts may have resulted from their reduced stability or failure to properly separate from the primary transcript. In contrast, when we converted the noncanonical, and much less common poly(A) signal (ATTAAA) to the canonical AATAAA (mutant 3; Fig. 4B), we eliminated both the primary and the mature llz transcripts. This result is consistent with derivation of mature llz transcripts from the primary transcript rather than from a cryptic promoter or some form of transcriptional reinitiation. In addition, these data suggest that the poly(A) signal of the upstream gene is important for preventing premature transcriptional termination at the locus. Why eukaryotes have evolved the less common, noncanonical ATTAAA poly(A) signal is not known (23). Our data suggest one potential function for this polyA signal.
CheB42a and llz Show Similar Expression Patterns.
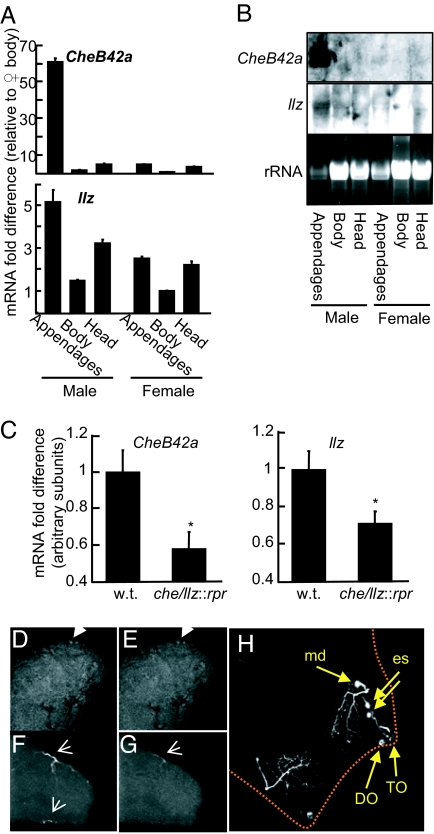
Operon-like transcription from a single promoter predicted the two genes would show similar expression patterns. A previous study reported that CheB42a was expressed in the front legs of males (5). In agreement with that work, real-time quantitative RT-PCR and Northern blot analyses revealed CheB42a enriched in male appendages (Fig. 5A and B); expression in females was less abundant. Importantly, llz showed the same pattern with enrichment in male appendages. Although the ratio of transcripts in appendages to those in bodies differed somewhat between CheB42a and llz, similar variations are observed with nematode operons (9). Such variations have been attributed to posttranscriptional processes that vary transcript levels for coordinately transcribed genes. We also found that ablating cells by expressing the apoptotic gene rpr (24) under control of the CheB42a/llz promoter reduced expression of both genes in male appendages (Fig. 5C). Together, these data suggest that both genes are expressed in male appendages.
Fig. 5.
Both CheB42a and llz transcripts are expressed preferentially in male-specific sensory structures. (A) Real-time quantitative RT-PCR. Transcripts of both CheB42a and llz were enriched in male appendages relative to other body parts or females by ANOVA (P < 0.001; n = 6 per group). Data are mean ± SEM. (B) Northern blot analysis of total RNA from adult tissues. The same membrane was probed for both genes with stripping between hybridizations. The blot of llz required much longer exposure of the membrane, suggesting lower-level expression than CheB42a. Data for male appendages are the same as those shown in Fig. 2A. (C) Genetic elimination of cells by expressing the cell death-inducer rpr under the CheB42a/llz promoter reduced abundance of both CheB42a and llz transcripts in male appendages (ANOVA P < 0.02; n = 4 per group). (D–G) In situ hybridization for CheB42a (D) and llz (E) in embryos. Individual embryos were hybridized simultaneously with CheB42a and llz antisense probes labeled with two different fluorophores or with the corresponding sense probes. The front of embryo is in upper right. Arrowheads indicate location of signal, likely in neurons. (F and G) CheB42a and llz sense controls. Arrows in F and G indicate nonspecific fluorescence in trachea. (H) The CheB42a-llz locus is expressed in third-instar larvae in a single lateral multidendritic neuron (md) plus two external sensory neurons (es) that project to the chemosensitive terminal organ (TO). Dorsal organ (DO), which shows autofluorescence, also is indicated. Larva is outlined by dotted line.
In situ hybridization of late-stage embryos localized signal in two cells in the anterior portion of embryos (Fig. 5 D–G); based on their position and rarity, these cells are likely to be neurons. Importantly, we saw exactly the same pattern with both CheB42a and llz probes, suggesting that both genes are expressed in the same cells.
To identify cells expressing the locus, we drove a GFP reporter with a CheB42a/llz promoter by using the UAS/GAL4 binary system (25). In the heads of third-instar larvae, we detected a class II multidendritic (mechanosensitive) neuron (26) and two external sensory neurons that project to the chemosensitive terminal organ (Fig. 5H). These data are consistent with data from the embryos and suggest that the locus might play a sensory role.
Discussion
Prokaryotes and nematodes use operon-based transcription to coordinately regulate genes participating in common biological pathways. Our data indicate that this elegant process also occurs in Drosophila. Although we have investigated only one gene pair in detail and obtained less data for two other gene pairs, our preliminary analysis suggests that the fly genome contains numerous gene pairs that may share a chromosomal arrangement like that of CheB42a/llz. It is likely that some of these also represent operon-like loci, an occurrence that would be consistent with the abundance of operons in the C. elegans genome. That genome has >15% of its coding genes transcribed as operons, many with already known functional relationships (7).
Bacterial and archaeal operons consist of a single RNA species from which multiple proteins are translated. Nematode operons also transcribe a pre-mRNA from a single promoter, but the primary transcript then is processed to individual mature transcripts for each gene (27). Processing involves trans splicing a specific short RNA sequence to each 5′ UTR (28). In fact, it was recognition of the specialized spliced leader (SL2) RNA attached to downstream transcripts that led to the discovery that C. elegans genomes contained operons (28). As in nematode operons, in the Drosophila CheB42a/llz locus, a single promoter produces a pre-mRNA that is converted posttranscriptionally to individual mRNAs. But Drosophila does not seem to exhibit SL RNA trans splicing, and, thus, the mechanism is distinct. Therefore, we speculate that processing of operons may have evolved separately in nematodes and Drosophila.
Our data provide some insight into how mature CheB42a and llz transcripts may be generated. Splitting of the pre-mRNA occurs after transcription. As in C. elegans operons, the poly(A) sequence and sequences in the intergenic region are important for production of both mature upstream and downstream transcripts. Especially interesting is our finding that the 5′ end of the downstream transcript appears to be formed without a conventional 5′ cap. Instead, we were surprised to find that llz transcripts contain a 5′ guanosine not encoded by DNA. We also found this feature on some transcripts from other potential operons; it may provide an important clue to discover how the 5′ end is formed. Thus, we speculate that the CheB42a/llz locus may use a nontraditional capping mechanism for the downstream gene. Interestingly, novel capping-like mechanisms recently have been described for other eukaryotic genes (29), and cap-independent translation has been reported in eukaryotes (16).
An operon-like organization for the CheB42a/llz locus suggested a functional relationship between the gene products. Consistent with this suggestion, CheB42a and llz showed a similar pattern of expression. Moreover, they both seem to be involved in male courtship behavior (5, 30, 31), and the two proteins interact (Y.B.-S. and M.J.W., unpublished data).
Recognition of operon-based transcription in Drosophila may allow a better understanding of its chromosomal organization and transcriptome. We also speculate that genomes of other eukaryotic species may contain similar genome-level architecture to coregulate gene expression. Finally, identification of operons in Drosophila and other higher eukaryotes might provide a new way to identify genes contributing to the same biochemical process or physiological pathway. As an example, the protein products of CheB42a and llz would never have been predicted to interact without the recognition of the operon-like genomic organization.
Materials and Methods
Drosophila Stocks and Cultures.
See SI Text for more information.
RNA Analysis.
Flies were separated by sex under CO2 and kept at −80°C until processing. To separate body parts, microcentrifuge tubes with flies were dipped in liquid nitrogen and then separated by repeated vortexing. Drosophila S2 cells were transfected with the Effectene reagent (Qiagen, Valencia, CA) according to manufacturer instructions. Total RNA from tissues or cells was extracted with the RNeasy mini kit (Qiagen) or TRizol reagent (Invitrogen, Carlsbad, CA) according to manufacturer instructions. RT-PCR analysis was performed by using the SuperScript II reverse transcriptase (Invitrogen) with 1 μg of total RNA in a 20-μl reaction according to manufacturer instructions. PCRs were performed with the ACCUprime pfx supermix (Invitrogen) in 25-μl reactions and were subsequently separated on a 1.2% agarose gel. Northern blots were performed with the Northern Max kit (Ambion, Austin, TX) and probed with DIG-labeled riboprobes (Roche, Indianapolis, IN). The same membranes were used for both probes and were stripped between hybridizations. Real-time quantitative RT-PCR assays were performed on an “ABI7000” or an “ABI7500 fast” with SYBR Green or PowerSybr as a reporter according to manufacturer instructions. For analysis, expression levels of the housekeeping gene rp49 were used as an RNA-loading control. Data were transformed according to the Δ Δ Ct method and are represented as relative values (32). Fold difference was calculated relative to the lowest-expressing sample, which represented 1 unit of expression. Primer sequences for all genes analyzed are contained in SI Table 2. The 5′ RACE analysis for capped transcripts was performed with the RLM-RACE kit (Ambion). 5′ RACE of noncapped mRNA was performed by using the cRACE protocol (33). Polysomal RNA extraction was performed as described in ref. 15 with the following modifications: Cells were treated with cyclohexamide for 15 min before lysis, and fractionated RNA was isolated with the TRIzol reagent (Invitrogen). Precipitation of capped mRNAs was performed with H2O anti-cap monoclonal antibody (Synaptic Systems) according to manufacturer's protocol. As a control, we incubated an aliquot of the same RNA with an unrelated antibody of identical isotype (IgG1). Both IPs were performed with protein-G Sepharose (Pierce) according to manufacturer's instructions. In situ hybridization analysis was performed as described in ref. 34; fluorescent probes were produced with the FISH-Tag kit (Invitrogen). Individual embryos were hybridized with a mixture of AlexaFluor 488-labeled CheB42a antisense and AlexaFluor 555-labeled llz antisense probes. For controls, individual embryos were labeled with the corresponding sense probes.
Operon Processing in S2 Cells.
The genomic region spanning all of the CheB42a/llz exons was PCR-amplified with ACCUprime pfx supermix (Invitrogen) and was subcloned into the metal-inducible pMT-V5-HIS vector (Invitrogen). The CheB42a transcriptional start site was determined according to the 5′ RACE data. Cells were transfected transiently with Effectene Transfection Reagent (Qiagen) according to manufacturer instructions. RNA was harvested from cells (RNeasy Mini Kit; Qiagen) after they were induced with 500 μM CuSO4 for 24 h.
Bioinformatics.
See SI Text for more information.
Supplementary Material
Acknowledgments
We thank Mariana Mata and Sarah Pedigo for help with fly maintenance and general laboratory assistance; Yuhong Li, Dan Collier and Nicholas Heiniger for help with experiments; the Univ. of Iowa In Vitro Models and Cell Culture Core for cell maintenance. This work was partly supported by the National Heart, Lung and Blood Institute, the Cystic Fibrosis Foundation Grants R458-CR02 and ENGLH9850, and National Institutes of Diabetes and Digestive and Kidney Diseases Grant DK54759. TES was partially supported by a Career Development Award from Research to Prevent Blindness. YBS is an Associate and MJW is an Investigator of the Howard Hughes Medical Institute.
Abbreviation
- RT
reverse transcription.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0609683104/DC1.
References
- 1.Bianchi L, Driscoll M. Neuron. 2002;34:337–340. doi: 10.1016/s0896-6273(02)00687-6. [DOI] [PubMed] [Google Scholar]
- 2.Krishtal O. Trends Neurosci. 2003;26:477–483. doi: 10.1016/S0166-2236(03)00210-8. [DOI] [PubMed] [Google Scholar]
- 3.Welsh MJ, Price MP, Xie J. J Biol Chem. 2002;277:2369–2372. doi: 10.1074/jbc.R100060200. [DOI] [PubMed] [Google Scholar]
- 4.Liu L, Leonard AS, Motto DG, Feller MA, Price MP, Johnson WA, Welsh MJ. Neuron. 2003;39:133–146. doi: 10.1016/s0896-6273(03)00394-5. [DOI] [PubMed] [Google Scholar]
- 5.Xu A, Park SK, D'Mello S, Kim E, Wang Q, Pikielny CW. Cell Tissue Res. 2002;307:381–392. doi: 10.1007/s00441-002-0524-0. [DOI] [PubMed] [Google Scholar]
- 6.Blumenthal T, Gleason KS. Nat Rev Genet. 2003;4:112–120. doi: 10.1038/nrg995. [DOI] [PubMed] [Google Scholar]
- 7.Blumenthal T, Evans D, Link CD, Guffanti A, Lawson D, Thierry-Mieg J, Thierry-Mieg D, Chiu WL, Duke K, Kiraly M, Kim SK. Nature. 2002;417:851–854. doi: 10.1038/nature00831. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence JG. Cell. 2002;110:407–413. doi: 10.1016/s0092-8674(02)00900-5. [DOI] [PubMed] [Google Scholar]
- 9.Lercher MJ, Blumenthal T, Hurst LD. Genome Res. 2003;13:238–243. doi: 10.1101/gr.553803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawrence JG. Cell. 2002;110:407–413. doi: 10.1016/s0092-8674(02)00900-5. [DOI] [PubMed] [Google Scholar]
- 11.Kuersten S, Lea K, MacMorris M, Spieth J, Blumenthal T. RNA. 1997;3:269–278. [PMC free article] [PubMed] [Google Scholar]
- 12.Andrews J, Smith M, Merakovsky J, Coulson M, Hannan F, Kelly LE. Genetics. 1996;143:1699–1711. doi: 10.1093/genetics/143.4.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitamoto T, Wang W, Salvaterra PM. J Biol Chem. 1998;273:2706–2713. doi: 10.1074/jbc.273.5.2706. [DOI] [PubMed] [Google Scholar]
- 14.Woodson SA. Curr Opin Struct Biol. 2005;15:324–330. doi: 10.1016/j.sbi.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Meyuhas O, Bibrerman Y, Pierandrie-Amaldi P, Amaldi F. In: A Laboratory Guide to RNA. Kreig PA, editor. New York: Wiley-Liss; 1996. pp. 65–81. [Google Scholar]
- 16.Merrick WC. Gene. 2004;332:1–11. doi: 10.1016/j.gene.2004.02.051. [DOI] [PubMed] [Google Scholar]
- 17.Hoehne M, de Couet HG, Stuermer CA, Fischbach KF. Mol Cell Neurosci. 2005;30:326–338. doi: 10.1016/j.mcn.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Morabito MA, Sheng M, Tsai LH. J Neurosci. 2004;24:865–876. doi: 10.1523/JNEUROSCI.4582-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han P, Dou F, Li F, Zhang X, Zhang YW, Zheng H, Lipton SA, Xu H, Liao FF. J Neurosci. 2005;25:11542–11552. doi: 10.1523/JNEUROSCI.3831-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Girardot N, Allinquant B, Langui D, Laquerriere A, Dubois B, Hauw JJ, Duyckaerts C. Neuropathol Appl Neurobiol. 2003;29:451–461. doi: 10.1046/j.1365-2990.2003.00479.x. [DOI] [PubMed] [Google Scholar]
- 21.Huang T, Kuersten S, Deshpande AM, Spieth J, MacMorris M, Blumenthal T. Mol Cell Biol. 2001;21:1111–1120. doi: 10.1128/MCB.21.4.1111-1120.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Huang T, MacMorris M, Blumenthal T. RNA. 2001;7:176–181. doi: 10.1017/s1355838201002333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacDonald CC, Redondo J-L. Mol Cell Endocrinol. 2002;190:1–8. doi: 10.1016/s0303-7207(02)00044-8. [DOI] [PubMed] [Google Scholar]
- 24.White K, Tahaoglu E, Steller H. Science. 1996;271:805–807. doi: 10.1126/science.271.5250.805. [DOI] [PubMed] [Google Scholar]
- 25.Phelps CB, Brand AH. Methods. 1998;14:367–379. doi: 10.1006/meth.1998.0592. [DOI] [PubMed] [Google Scholar]
- 26.Grueber WB, Jan LY, Jan YN. Cell. 2003;112:805–818. doi: 10.1016/s0092-8674(03)00160-0. [DOI] [PubMed] [Google Scholar]
- 27.Hastings KEM. Trends Genet. 2005;21:240–247. doi: 10.1016/j.tig.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Spieth J, Brooke G, Kuersten S, Lea K, Blumenthal T. Cell. 1993;73:521–532. doi: 10.1016/0092-8674(93)90139-h. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen H, Westhof E, Johansen S. Science. 2005;309:1584–1587. doi: 10.1126/science.1113645. [DOI] [PubMed] [Google Scholar]
- 30.Lin H, Mann KJ, Starostina E, Kinser RD, Pikielny CW. Proc Natl Acad Sci USA. 2005;102:12831–12836. doi: 10.1073/pnas.0506420102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park SK, Mann KJ, Lin H, Starostina E, Kolski-Andreaco A, Pikielny CW. Curr Biol. 2006;16:1154–1159. doi: 10.1016/j.cub.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 32.Ben-Shahar Y, Robichon A, Sokolowski MB, Robinson GE. Science. 2002;296:741–744. doi: 10.1126/science.1069911. [DOI] [PubMed] [Google Scholar]
- 33.Maruyama IN, Rakow TL, Maruyama HI. Nucleic Acids Res. 1995;23:3796–3797. doi: 10.1093/nar/23.18.3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kosman D, Mizutani CM, Lemons D, Cox WG, McGinnis W, Bier E. Science. 2004;305:846. doi: 10.1126/science.1099247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.