Abstract
Signaling in the nervous system requires matching of neurotransmitter receptors with cognate neurotransmitters at synapses. The vertebrate neuromuscular junction is the best studied cholinergic synapse, but the mechanisms by which acetylcholine is matched with acetylcholine receptors are not fully understood. Because alterations in neuronal calcium spike activity alter transmitter specification in embryonic spinal neurons, we hypothesized that receptor expression in postsynaptic cells follows changes in transmitter expression to achieve this specific match. We find that embryonic vertebrate striated muscle cells normally express receptors for glutamate, GABA, and glycine as well as for acetylcholine. As maturation progresses, acetylcholine receptor expression prevails. Receptor selection is altered when early neuronal calcium-dependent activity is perturbed, and remaining receptor populations parallel changes in transmitter phenotype. In these cases, glutamatergic, GABAergic, and glycinergic synaptic currents are recorded from muscle cells, demonstrating that activity regulates matching of transmitters and their receptors in the assembly of functional synapses.
Keywords: calcium spikes, homeostatic receptor plasticity, neurotransmitters, spinal cord, skeletal muscle
Information processing in the nervous system relies on transmission of electrical signals at synapses via release of presynaptic neurotransmitters that bind to neurotransmitter receptors on postsynaptic cells. Matching of transmitter receptors with transmitters is critical for signaling by networks of neurons, because mismatch would disrupt information transfer. Evaluation of mechanisms that regulate expression of transmitters and receptors suggests that neuronal activity may be an important factor. Calcium-dependent electrical activity in Xenopus spinal neurons homeostatically regulates specification of acetylcholine (ACh), glutamate, GABA, and glycine at early stages of development (1), depolarization increases the proportion of dopaminergic neurons in nodose, petrosal, and dorsal root rat ganglia (2), and specification of GABAergic and glutamatergic phenotypes in developing hippocampal rat granule cells depends on electrical activity (3). NMDA receptor (NMDAR) expression is modulated by early activity in the rat superior colliculus (4) and in cat and ferret visual cortex (5), and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) levels are activity-dependent in postnatal rat nucleus accumbens neurons (6). Kainate receptor expression depends on activity in postnatal rat thalamocortical synapses (7). Electrical activity also regulates subunit expression of GABAA receptors (GABAARs) in rat superior colliculus neurons (8) and ACh receptors (AChRs) at the neonatal mouse neuromuscular junction (NMJ) (9).
The transmitter acetylcholine and acetylcholine receptors are normally paired at the vertebrate NMJ (10–12), and genetic programs regulate aspects of differentiation of motor neurons and muscle cells (13, 14). However, several features of synapse formation have been shown to be activity-dependent (15–18), and presynaptic and postsynaptic differentiation appear to be mutually inductive events. For example, presynaptically derived agrin serves as the trigger for the localization of receptors and the associated postsynaptic machinery in muscle cells (19). Before neuronal contact, AChRs are distributed over the entire surface of the muscle cell, with occasional high-density patches. After neuromuscular contact, AChRs cluster rapidly in the postsynaptic membrane, and the density of extrajunctional AChRs decreases gradually in a process controlled by muscle cell activity (20–22). The role of neurotransmission in NMJ formation has been tested by generating mutant mice lacking activity of the ACh-synthesizing enzyme choline acetyltransferase; the numbers of all three synaptic components (motor axons, myotubes, and Schwann cells) were affected (16). Retrograde signaling also regulates development of the NMJ. Before establishing terminal synapses with their final muscle targets, migrating motor axons form en passant synaptic contacts with myotomal muscle in zebrafish embryos. In twister AChR mutants, neuromuscular transmission is prolonged at these synapses, giving rise to aberrant motor axon trajectories as well as muscular degeneration (23). In Caenorhabditis elegans muscle cells appear to be required for formation of presynaptic varicosities by innervating motoneurons (24). Although these studies clearly define the importance of neural activity and bidirectional interactions in the assembly and refinement of a newly formed synapse, little is known regarding the mechanisms of matching a transmitter with the appropriate class of receptors.
Here, we show that embryonic muscle cells initially express several classes of transmitter receptors in addition to those for ACh. During normal differentiation and innervation of muscle, the other classes of receptors disappear. Changing the expression of transmitters by altering calcium spike activity leads to retention of the classes of cognate, noncholinergic receptors. Under these conditions, we record glutamatergic, GABAergic, and glycinergic synaptic currents from the skeletal muscle, as well as those mediated by nicotinic AChRs. We find that selection of appropriate receptors depends on diffusible factors from neurons. The results indicate that early neuronal activity ensures matching of transmitters and their receptors and regulates the identity of newly formed synapses.
Results
Developmental Regulation of Neurotransmitter Receptor Expression in Skeletal Muscle.
We analyzed developmental regulation of expression of transmitter receptor transcripts in muscle by RT-PCR. Innervation of Xenopus axial musculature starts at 1 day after fertilization, and the embryo progresses through an early flexure stage to a free swimming stage that starts at 2 days (25, 26). We identify mRNA encoding subunits of receptors for ACh (nAChR), glutamate (NMDAR and AMPAR), GABA (GABAAR), and glycine (glycine receptor; GlyR) in axial muscle at 1 day (stage 22; Fig. 1A Top). These transcripts are still present at 1.3 days (stage 28; Fig. 1A Middle), but only those encoding nAChRα1 and NMDAR NR1 (faint band) are detected by 3 days of normal development (stage 40; Fig. 1A Bottom). All transcripts are present in the neural tube throughout this period and absent from the notochord at the early stages. Early neuronal markers NeuroD and neurogenin-related-protein-1 are amplified from neural tube but not from muscle or notochord; thus, contamination of muscle by transcripts from the neural tube is unlikely.
Fig. 1.
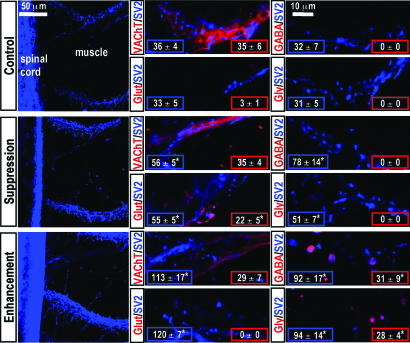
Expression of nAChR, NMDAR, AMPAR, GABAAR, and GlyR transcripts and protein in skeletal muscle during normal development and after alterations in neuronal activity. (A) RT-PCR was used for detection of subunit transcripts of five neurotransmitter receptors in muscle, notochord, and neural tube at three stages of development. Tissue-specific RNA was analyzed from embryos at 1 day (stage 22, Top), at 1.3 days (stage 28, Middle) and from larvae at 3 days (stage 40, Bottom). Primers were designed from predicted Xenopus sequences for nAChRα1, NR1, GluR1, GABAARβ2, and GlyRα1 subunits and for neuronal markers NeuroD and neurogenin-related-protein-1 (NGNR-1). (B and C) Multiple classes of transmitter receptors are expressed in embryonic skeletal muscle in vivo. Whole mounts from 1.3-day (stage 28) embryos and 3-day (stage 40) larvae were labeled for myosin and nuclei (B), nAChR, NMDAR, AMPAR, GABAAR, and GlyR (C) with probes noted above each column. Images of chevrons of mononucleate muscle cells are representative Z series projections obtained from confocal stacks of 20 optical sections of 62,500 μm2 area. (C Insets) Percent of labeled volume. Values are mean ± SEM, n ≥ 5 embryos for each probe. ∗, P < 0.001 when compared with stage 40 for each probe. (D) Alterations of neuronal Ca2+ spike activity change in vivo expression of transmitter receptors in larval skeletal muscle. Whole mounts from activity-manipulated 3-day (stage 40) larvae were labeled for transmitter receptors as in C. Manipulation of activity was achieved by implanting beads impregnated with 30 μM tetrodotoxin, 200 nM calcicludine, 10 μM GVIA ω-conotoxin, and 10 μM flunarizine (Upper, Ca2+ spike activity suppression) or with 1 mM veratridine (Lower, Ca2+ spike activity enhancement). Specimens were stained and labeling was quantified (Insets) as in C. Values are mean ± SEM for n ≥ 5 embryos for each probe. ∗, P < 0.001 when compared with stage 40 control for each probe.
We then assessed the expression of transmitter receptor proteins by evaluating agonist and antagonist binding and receptor subunit immunohistochemistry of permeabilized muscle (Fig. 1B). We find that nAChR levels increase from 1.3 to 3 days of development, as previously described (27). NMDARs, AMPARs, GABAARs, and GlyRs are present initially, and their levels decrease during this period (Fig. 1C). Labeling of these receptors appears to be specific. Antibodies used to characterize NR1, AMPAR GluR1, and GlyRα1 subunits in muscle identify bands of predicted size on Western blots. Controls performed with antigen-absorbed antibodies and competitive antagonists or unlabeled ligands for nAChRs and GABAARs yield staining not different from background [supporting information (SI) Fig. 5]. Labeling of a constant area and depth quantifies the developmental changes in total intracellular and surface receptor levels (Fig. 1C Insets). These results indicate that vertebrate skeletal muscle expresses five classes of neurotransmitter receptors at early developmental stages, four of which are selectively eliminated to achieve the mature cholinergic phenotype.
Changes in Early Neuronal Activity Alter Developmental Progression of Neurotransmitter Receptor Expression.
We next altered neuronal calcium (Ca2+) spike activity to test whether changes in transmitter specification in embryonic spinal neurons perturb the selection of transmitter receptors in skeletal muscle. Activity was suppressed or enhanced by implanting agarose beads adjacent to the neural tube at 20 h of development, loaded with Ca2+ and sodium (Na+) channel blockers or veratridine, a voltage-gated Na+ channel agonist (1). When suppressing activity and increasing the incidence of glutamatergic and cholinergic neurons (1), we find a significant shift in the distribution of nAChRs from junctional to extrajunctional regions at 3 days of development, although the overall expression in axial musculature does not change (Fig. 1D). Enhancing activity decreases expression of nicotinic receptors. These results are consistent with observations of the effects of the presence and absence of electrical activity on the distribution of nAChRs in adult muscle (28, 29). NMDAR and AMPAR expression increases when activity is suppressed and decreases when activity is enhanced (Fig. 1D). Expression of inhibitory transmitter receptors, GABAARs and GlyRs, does not change significantly when activity is suppressed but increases when activity is enhanced, a condition that causes an increase in the incidence of GABAergic and glycinergic neurons (1) (Fig. 1D). These findings demonstrate activity-dependent regulation of expression of different classes of transmitter receptors in skeletal muscle that parallels changes in transmitter specification in developing spinal neurons.
Noncholinergic Terminals Project to Muscle When Early Neuronal Activity Is Altered.
To assess anatomically the potential noncholinergic innervation of the axial musculature after manipulation of early neuronal activity, we labeled whole-mount preparations for neurotransmitter phenotype and a presynaptic marker, SV2. The number of synaptic puncta in axial musculature of larvae in which activity has been suppressed or enhanced is greater than in controls (Fig. 2Left). In control larvae, nerve terminals stain strongly for the vesicular ACh transporter (VAChT), weakly for glutamate, and not for GABA or glycine. After Ca2+ spike suppression, staining for VAChT endures but some axons and presynaptic boutons are strongly stained for glutamate and not for GABA or glycine. VAChT staining also persists after Ca2+ spike enhancement, but glutamate staining is absent, and some boutons are now stained for GABA or glycine (Fig. 2 Center and Right). Antibodies to VAChT appear to stain both axons and terminal varicosities, whereas the antibodies to glutamate, GABA, and glycine appear to stain terminal varicosities alone. Although different antibody affinities and permeabilities may contribute to this profile, these results suggest that cholinergic innervation remains abundant after perturbation of Ca2+ spikes and the appearance of noncholinergic transmitters. Labeling of a constant area quantifies the numbers of SV2- and transmitter-labeled puncta for each condition (Fig. 2 Insets). These results show that perturbations of early neuronal activity increase the number of synaptic puncta and suggest that they influence the class of transmitter in axons innervating skeletal muscle.
Fig. 2.
Activity-dependent neurotransmitter expression in presynaptic terminals in the axial musculature. Whole mounts from control and activity-manipulated 3-day (stage 40) larvae were immunolabeled for a presynaptic marker, SV2 (in blue), and for transmitter phenotype (in red): VAChT (cholinergic), glutamate (glutamatergic), GABA (GABAergic), and glycine (glycinergic). (Left) Low magnification images are representative Z series projections obtained from confocal stacks of 50 optical sections of 44,100 μm2 area of muscle and spinal cord. (Center and Right) High magnification images are representative Z series projections of five optical sections of muscle from the top of the same stacks. n = 5 embryos for each transmitter phenotype. (Insets) Numbers of synaptic puncta labeled for SV2 and for transmitter per 33,443 μm2 area of muscle. Values are mean ± SEM for n = 5 single optical sections per probe from five embryos. ∗, P < 0.05 compared with control.
Noncholinergic Neuromuscular Junctions Are Established When Early Neuronal Activity Is Manipulated.
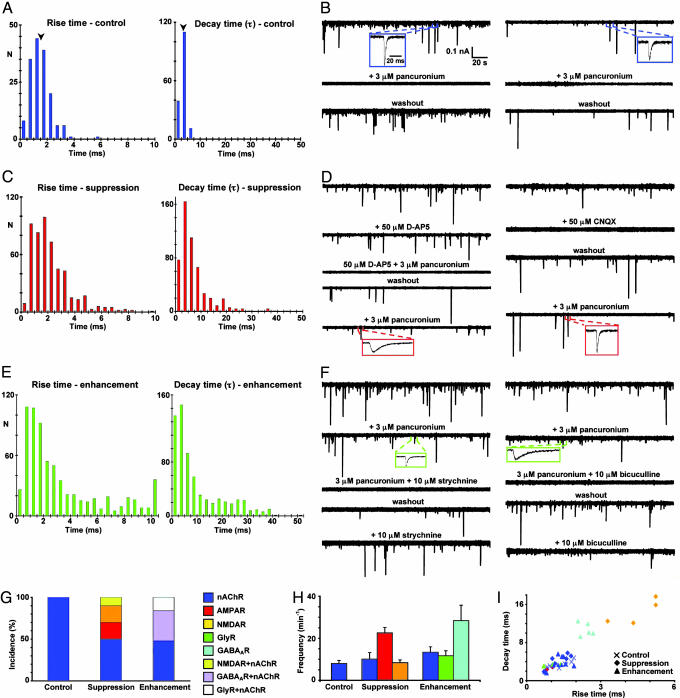
To determine whether NMJs are formed by using glutamate, GABA, or glycine as transmitters, we first recorded miniature postsynaptic currents (mpscs) from muscle cells in control 3-day larvae. Excitatory mpscs (mepscs) occurred at a mean frequency of 7 min−1 (range 3–11 min−1; n = 8 NMJs). Histograms of kinetic parameters of these mepscs show a tight distribution of rapid rise and decay times, with decays that are fit by a single exponential (Fig. 3A and SI Fig. 6), suggesting that a single receptor type is involved. These mepscs were completely blocked by 3 μM pancuronium (n = 6), a nAChR antagonist, identifying them as cholinergic, consistent with previous results (25, 26) (Fig. 3 B, G and H). Events blocked by pancuronium exhibit rise and decay times (Fig. 3I), similar to those described for nicotinic receptor-mediated currents (30).
Fig. 3.
Matching of neurotransmitters and receptors at neuromuscular junctions in vivo. Whole-cell recordings from muscle cells of the axial musculature of 3-day (stage 40) control (A and B), Ca2+ spike-suppressed (C and D), and Ca2+ spike-enhanced (E and F) larvae were performed in the presence of 2 mM Ca2+, Mg2+-free saline, and 3 μM TTX; Vh = −80 mV. (A, C, and E) Rise and decay time distributions for mpscs, including only mpscs with decay times fit by single exponentials. N, number of mpscs (≥6 embryos for each group). Arrowheads in A indicate mean values. (B, D, and F) Examples of pharmacological blockade of mpscs from two muscle cells in each group of embryos. Single mpscs are shown on an expanded time base to illustrate their kinetics. (G) Incidence of cholinergic, glutamatergic, GABAergic, and glycinergic NMJs. Bars represent the percent of each class of NMJ in each group based on blockade by different receptor antagonists. Bars represented by a single receptor are derived from NMJs in which mpscs were blocked completely by a single receptor antagonist. Bars corresponding to two receptors are derived from NMJs in which mpscs were blocked completely only by the combination of two receptor antagonists. (H) Frequencies of pharmacologically isolated mpscs in each group. Values are mean ± SEM. (I) Rise and decay times for pharmacologically identified mpscs. ×, control; ◇, spike-suppressed; ▵, spike-enhanced. Each point is the mean rise time and decay time for mpscs recorded from a single muscle cell. (G–I) Values are from 6 control, 12 spike-suppressed, and 13 spike-enhanced NMJs.
We next recorded mpscs from muscle cells in larvae that had been implanted embryonically with beads to alter neuronal Ca2+ spike activity. After suppressing Ca2+ spike activity, the distribution of rise and decay times is broader, and some events are fit by two exponentials, suggesting several receptor types are involved (Fig. 3C and SI Fig. 6). These mepscs occurred at a mean frequency of 24 min−1 (range 3–112 min−1; significantly different from control, P < 0.005; n = 26). Examination of 12 NMJs from 10 larvae yielded mepscs that were blocked by glutamate receptor antagonists at six junctions. Some were blocked by 50 μM D-AP5, an NMDAR antagonist, and others by 50 μM CNQX, an AMPAR antagonist, indicating the presence of glutamatergic currents that were kinetically similar to those recorded from spinal neurons (30, 31) (Fig. 3 D and G–I). Occasionally, blockade was complete only when nicotinic and glutamatergic antagonists were added together, indicating both cholinergic and glutamatergic innervation of a single muscle cell (Fig. 3 D and G). The proportion of excitatory noncholinergic responses after Ca2+ spike suppression is significantly different from the control (P < 0.05, Fisher's exact test).
After loading beads with veratridine, histograms of kinetic parameters again exhibit a broad distribution of rise and decay times, and some events are fit by two exponentials, suggesting the presence of several classes of receptors (Fig. 3E and SI Fig. 6). These mpscs were generated at a frequency of 34 min−1 (range 5–179 min−1; significantly different from control, P < 0.001; n = 27). Examination of 13 NMJs from 10 larvae yielded inhibitory mpscs (mipscs) at 6 junctions that were blocked by 10 μM bicuculline or 10 μM strychnine, GABAAR, and GlyR blockers (Fig. 3 F–I). These results demonstrate the presence of currents that are kinetically similar to those recorded from spinal neurons (32). Mipscs were interleaved with cholinergic mepscs. The proportion of inhibitory responses after Ca2+ spike enhancement is significantly different from the control (P < 0.05, Fisher's exact test). Forty percent of muscle cells exhibited only cholinergic innervation after either suppression or enhancement of neuronal Ca2+ spike activity (Fig. 3G). However, altering neuronal Ca2+ spike activity leads to the appearance of NMJs with novel classes of mpscs, which are generated at different frequencies (Fig. 3H).
Activity-Dependent Neuronal Diffusible Factors Signal to Muscle Cells and Regulate Neurotransmitter Receptor Expression.
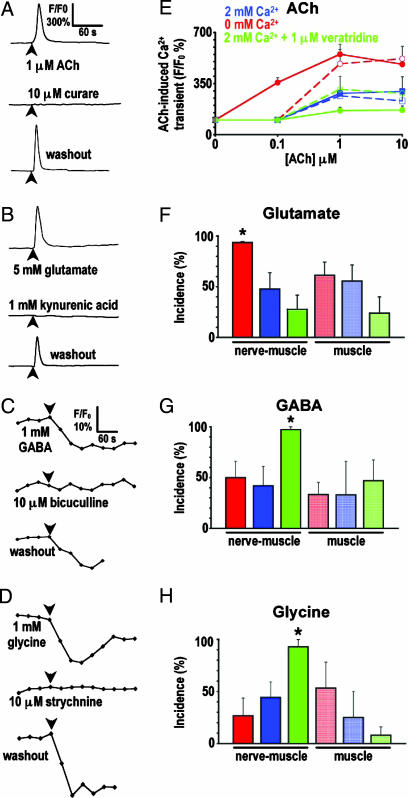
To begin to analyze the mechanism underlying transmitter-receptor matching, we determined whether embryonic skeletal muscle cells in dissociated cell culture express different classes of receptors, using an imaging screen to test the transmitter sensitivity of muscle cells cocultured with neurons. Uninnervated muscle cells loaded with Fluo-4AM, a fluorescent Ca2+ indicator, demonstrated transient elevations of intracellular Ca2+ in response to 1 μM ACh or 5 mM glutamate. ACh responses were blocked by 3 μM pancuronium, and 80% of glutamate responses were blocked by 1 mM kynurenate, an ionotropic glutamate receptor antagonist (Fig. 4A and B). Increases in intracellular chloride (Cl−) were recorded in response to 1 mM GABA or 1 mM glycine when cells were loaded with diH-MEQ, a fluorescent Cl− reporter. These responses were blocked by 10 μM bicuculline and 10 μM strychnine, respectively (Fig. 4 C and D). Thus, the excitatory and inhibitory transmitter receptors observed in vivo are functional in embryonic skeletal muscle cells grown in vitro.
Fig. 4.
Sensitivity of uninnervated muscle cells to ACh, glutamate, GABA, and glycine depends on neuronal Ca2+ spike activity in vitro. Muscle cells cocultured with neurons (nerve-muscle) and muscle cells cultured alone (muscle) for 18–24 h were loaded with a fluorescent Ca2+ indicator or Cl− sensor dye to image responses to ACh and glutamate or GABA and glycine, respectively. Imaging was performed in 2 mM Ca2+ culture medium. Glutamate responses were recorded in the presence of 10 μM curare or 3 μM pancuronium to exclude indirect nAChR-mediated responses. (A–D) Traces are responses of uninnervated single muscle cells to neurotransmitters applied at times indicated by arrowheads. (E–H) Analysis of the incidence of responses to transmitters under different culture conditions. ∗, P < 0.01 when compared with the 2 mM Ca2+ condition for nerve-muscle cultures. (E) Solid lines represent nerve-muscle cultures, and dashed lines represent muscle cultures. Values are mean ± SEM from >40 cells from five independent cultures for each transmitter.
To evaluate the dependence of transmitter sensitivity of muscle cells on neuronal activity, we grew nerve-muscle cocultures and cultures of muscle cells alone under different conditions. Growth in the absence of extracellular Ca2+ or in the presence of 2 mM Ca2+ and 1 μM veratridine suppressed or enhanced the generation of neuronal Ca2+ spikes but had no effect on spontaneous generation of Ca2+ transients in muscle cells (33) (SI Fig. 7). The transmitters expressed in the neurons changed in response to these changes in Ca2+ spike activity (SI Fig. 8) as reported for neurons cultured in the absence of muscle cells (1). Analysis of spontaneous mpscs in innervated muscle cells grown under these conditions revealed that noncholinergic NMJs are formed after perturbations of spontaneous neuronal Ca2+ spike activity (SI Fig. 9) as observed in vivo.
Uninnervated and uncontacted muscle cells exhibited differences in the incidence of responses to different transmitters depending on the culture conditions. These muscle cells invariably generated Ca2+ elevations in response to ACh, but their sensitivity was greatest when cocultures were grown in Ca2+-free medium (Fig. 4E) that increases the incidence of cholinergic neurons (SI Fig. 8). The high level of ACh sensitivity was absent when muscle cells were cultured in the absence of neurons. The incidence of muscle cells responsive to glutamate exhibited a similar dependence on neuronal activity (Fig. 4F), paralleling the differentiation of more glutamatergic neurons (SI Fig. 8). The incidence of responsiveness to GABA and glycine showed a reverse dependence on neuronal activity. Cocultures grown in the presence of Ca2+ plus veratridine, which leads to a greater number of GABAergic and glycinergic neurons (SI Fig. 8), demonstrated the highest incidence of muscle cells responsive to GABA and to glycine; this effect was absent when neurons were omitted from the cultures (Fig. 4 G and H). Because the effects observed in nerve-muscle cocultures depend on neuronal Ca2+ spike activity, these data suggest that neurons signal to muscle cells by diffusible factors indicating the class of transmitter they express, which specify selection of the corresponding receptors.
Discussion
These results show that alterations in neuronal Ca2+ spike activity lead to matching of NMDARs, AMPARs, GABAARs, GlyRs, and nAChRs with their cognate neurotransmitters at the vertebrate NMJ. Thus, activity can ensure formation of functional synapses, and the class of synapse depends on the level of this activity. We infer that presynaptic changes in transmitter phenotype drive postsynaptic changes in neurotransmitter receptors. Perturbations of neuronal Ca2+ spike activity do not affect generation of Ca2+ transients in muscle cells, and the pattern of changes in transmitter receptors on muscle cells follows the pattern of homeostatic changes in transmitter specification in neurons. The cell culture results argue for a mechanism involving diffusible factors that may include, but not be limited to, the transmitters themselves. The appearance of mpscs entirely mediated by inhibitory transmitters in vitro that are not observed in vivo (cf. SI Fig. 9 and Fig. 3G) may be due to the absence of guidance constraints on innervation in culture. Whether multimodal mpscs are due to corelease of several transmitters from the same vesicle or release from distinct populations of vesicles in the same or different nerve terminals remains to be determined. The results are in agreement with findings of glutamatergic transmission at adult vertebrate NMJs (34) and with the release of glutamate from neonatal mammalian motorneurons (35, 36). Glutamatergic-cholinergic cotransmission at the vertebrate NMJ may contribute to apparently normal behavioral and physiological development after chronic blockade of embryonic electrical activity (37, 38).
The expression of several classes of transmitter receptor in muscle early in development resembles the expression of receptors in neurons, most of which express more than one class. The mechanisms by which neurons localize appropriate receptors opposite the terminals releasing particular transmitters may be similar to the mechanisms involved in transmitter-receptor matching at the NMJ. Activity may play a critical role in sorting different classes of terminals with their cognate postsynaptic receptors in developing neuronal synapses (39, 40).
The expression of glutamate and GABA receptors in vertebrate skeletal muscle is reminiscent of the expression of these receptors at invertebrate NMJs of crabs, lobsters, locusts, and nematodes. In turn, invertebrate skeletal muscle express AChRs in nematodes, echinoderms, and molluscs. Given a common ancestry, it is perhaps unsurprising to observe expression of these noncholinergic receptors in vertebrate skeletal muscle. The observation of glycinergic vertebrate NMJs remains intriguing, because we are not aware of invertebrate NMJs that use glycine as a transmitter. The richness of transmitter receptor expression in muscle may be relatively unexplored.
We propose a model in which multiple classes of neurotransmitter receptors are expressed initially and the appropriate populations are selected to achieve transmitter-receptor matching observed at mature synapses. Receptors that are activated may become stabilized, whereas those that are not are either inactivated or removed (41–43), and transmitter receptor genes may be differentially regulated (14, 44, 45). Tests of the model will include simultaneous tracking of transmitter expression and clustering of cognate with disappearance of noncognate receptors and identification of the relevant diffusible factors. Embryonic neurons of other species generate developmentally transient Ca2+-dependent action potentials (46–49) that may produce Ca2+ spikes like those described for Xenopus spinal neurons and regulate transmitter and receptor expression similarly.
Methods
RT-PCR.
RT-PCR was performed on cDNA synthesized from tissue-specific isolated RNA. Primers were designed to amplify predicted sequences for Xenopus nAChRα1, NMDAR NR1, AMPAR GluR1, GABAARβ2, and GlyRα1 because these receptor subunits are widely expressed in the nervous system. NeuroD and neurogenin-related-protein-1 were amplified as neuron-specific markers. Further details are described in SI Methods and Table 1.
Labeling of Neurotransmitter Receptors and Western Blots.
Whole-mount preparations were incubated with 1 μM Sytox Green (a nuclear marker; Molecular Probes, Eugene, OR) and with MF20 antibody to myosin (Developmental Studies Hybridoma Bank, Iowa City, IA) to confirm muscle cell identity. Receptors were labeled with 5 μg/ml Alexa 488-α-bungarotoxin, 100 nM BODIPY-TMR-muscimol (Molecular Probes), or antibodies to GlyRα1, NR1, or GluR1 (Chemicon, Temecula, CA). Western blots were carried out on membrane protein extracts of whole larvae (stage 40) with the antibodies used for immunohistochemistry. See SI Methods for further details.
Labeling of Neurotransmitters in Presynaptic Nerve Terminals.
Stage 40 larvae were fixed and the spinal cord and the innervated myotome were exposed to antibodies to the VAChT (Chemicon), glutamate (Sigma, St. Louis, MO), and GABA and glycine (Chemicon) and to a presynaptic marker, SV2 (Developmental Studies Hybridoma Bank). See SI Methods for further details.
Electrophysiology.
Recording techniques and analysis are described in SI Methods.
Imaging and Neurotransmitter Immunocytochemistry in Vitro.
See SI Methods for further details.
Statistical Analysis.
Student's t test was used to assess significance of differences between experimental and control data unless otherwise indicated.
Supplementary Material
Acknowledgments
We thank Darwin Berg, Marla Feller, Kurt Marek, and Cory Root for comments on the manuscript and I-Teh Hsieh for technical support. This work was supported by a grant from the National Institutes of Health (to N.C.S.) and the National Science Foundation (to L.N.B.).
Abbreviations
- ACh
acetylcholine
- AChR
ACh receptor
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- GABAAR
GABAA receptor
- GlyR
glycine receptor
- mpsc
miniature postsynaptic current
- mepsc
excitatory mpsc
- NMDAR
NMDA receptor
- NMJ
neuromuscular junction
- VAChT
vesicular acetylcholine transporter.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0607450104/DC1.
References
- 1.Borodinsky LN, Root CM, Cronin JA, Sann SB, Gu X, Spitzer NC. Nature. 2004;429:523–530. doi: 10.1038/nature02518. [DOI] [PubMed] [Google Scholar]
- 2.Brosenitsch TA, Katz DM. Mol Cell Neurosci. 2002;20:447–457. doi: 10.1006/mcne.2002.1135. [DOI] [PubMed] [Google Scholar]
- 3.Gomez-Lira G, Lamas M, Romo-Parra H, Gutierrez R. J Neurosci. 2005;25:6939–6946. doi: 10.1523/JNEUROSCI.1674-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi J, Townsend M, Constantine-Paton M. Neuron. 2000;28:103–114. doi: 10.1016/s0896-6273(00)00089-1. [DOI] [PubMed] [Google Scholar]
- 5.Catalano SM, Chang CK, Shatz CJ. J Neurosci. 1997;17:8376–8390. doi: 10.1523/JNEUROSCI.17-21-08376.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mangiavacchi S, Wolf ME. Eur J Neurosci. 2004;20:649–657. doi: 10.1111/j.1460-9568.2004.03511.x. [DOI] [PubMed] [Google Scholar]
- 7.Kidd FL, Isaac JT. Nature. 1999;400:569–573. doi: 10.1038/23040. [DOI] [PubMed] [Google Scholar]
- 8.Henneberger C, Juttner R, Schmidt SA, Walter J, Meier JC, Rothe T, Grantyn R. Eur J Neurosci. 2005;21:431–440. doi: 10.1111/j.1460-9568.2005.03869.x. [DOI] [PubMed] [Google Scholar]
- 9.Missias AC, Chu GC, Klocke BJ, Sanes JR, Merlie JP. Dev Biol. 1996;179:223–238. doi: 10.1006/dbio.1996.0253. [DOI] [PubMed] [Google Scholar]
- 10.Dale HH, Feldberg W, Vogt M. J Physiol. 1936;86:353–380. doi: 10.1113/jphysiol.1936.sp003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fatt P, Katz B. J Physiol. 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- 12.Diamond J, Miledi R. J Physiol. 1962;162:393–408. doi: 10.1113/jphysiol.1962.sp006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL. Cell. 1994;79:957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 14.Piette J, Bessereau JL, Huchet M, Changeux JP. Nature. 1990;345:353–355. doi: 10.1038/345353a0. [DOI] [PubMed] [Google Scholar]
- 15.Poo MM. Phosphoprotein Res. 1994;29:521–527. doi: 10.1016/s1040-7952(06)80033-9. [DOI] [PubMed] [Google Scholar]
- 16.Misgeld T, Burgess RW, Lewis RM, Cunningham JM, Lichtman JW, Sanes JR. Neuron. 2002;36:635–648. doi: 10.1016/s0896-6273(02)01020-6. [DOI] [PubMed] [Google Scholar]
- 17.Hanson MG, Landmesser LT. Neuron. 2004;43:687–701. doi: 10.1016/j.neuron.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Kavalali ET, Kligauf J, Tsien RW. Proc Natl Acad Sci USA. 1999;96:12893–12900. doi: 10.1073/pnas.96.22.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanes JR, Lichtman JW. Nat Rev Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- 20.Schuetze SM, Role LW. Annu Rev Neurosci. 1987;10:403–457. doi: 10.1146/annurev.ne.10.030187.002155. [DOI] [PubMed] [Google Scholar]
- 21.Hall ZW, Sanes JR. Cell. 1993;72(Suppl):99–121. doi: 10.1016/s0092-8674(05)80031-5. [DOI] [PubMed] [Google Scholar]
- 22.Panzer JA, Song Y, Balice-Gordon RJ. J Neurosci. 2006;26:934–947. doi: 10.1523/JNEUROSCI.3656-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lefebvre JL, Ono F, Puglielli C, Seidner G, Franzini-Armstrong C, Brehm P, Granato M. Development (Cambridge, UK) 2004;131:2605–2618. doi: 10.1242/dev.01123. [DOI] [PubMed] [Google Scholar]
- 24.Plunkett JA, Simmons RB, Walthall WW. Dev Biol. 1996;175:154–165. doi: 10.1006/dbio.1996.0103. [DOI] [PubMed] [Google Scholar]
- 25.Blackshaw S, Warner A. Nature. 1976;262:217–218. doi: 10.1038/262217a0. [DOI] [PubMed] [Google Scholar]
- 26.Kullberg RW, Lentz TL, Cohen MW. Dev Biol. 1977;60:101–129. doi: 10.1016/0012-1606(77)90113-0. [DOI] [PubMed] [Google Scholar]
- 27.Goldfarb J, Cantin C, Cohen MW. J Neurosci. 1990;10:500–507. doi: 10.1523/JNEUROSCI.10-02-00500.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miledi R. J Physiol. 1960;151:1–23. [PMC free article] [PubMed] [Google Scholar]
- 29.Lomo T, Rosenthal J. J Physiol. 1972;221:493–513. doi: 10.1113/jphysiol.1972.sp009764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li W-C, Soffe SR, Roberts A. Proc Natl Acad Sci USA. 2004;101:15488–15493. doi: 10.1073/pnas.0404864101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rohrbough J, Spitzer NC. J Neurosci. 1999;19:8528–8541. doi: 10.1523/JNEUROSCI.19-19-08528.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jonas P, Bischofberger J, Sandkuhler J. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- 33.Ferrari MB, Rohrbough JC, Spitzer NC. Dev Biol. 1996;178:484–497. doi: 10.1006/dbio.1996.0233. [DOI] [PubMed] [Google Scholar]
- 34.Brunelli G, Spano P, Barlati S, Guarneri B, Barbon A, Bresciani R, Pizzi M. Proc Natl Acad Sci USA. 2005;102:8752–8757. doi: 10.1073/pnas.0500530102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishimaru H, Restrepo CE, Ryge J, Yanagawa Y, Kiehn O. Proc Natl Acad Sci USA. 2005;102:5245–5249. doi: 10.1073/pnas.0501331102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mentis GZ, Alvarez FJ, Bonnot A, Richards DS, Gonzalez-Forero D, Zerda R, O'Donovan MJ. Proc Natl Acad Sci USA. 2005;102:7344–7349. doi: 10.1073/pnas.0502788102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrison RG. Am J Anat. 1904;3:197–220. [Google Scholar]
- 38.Haverkamp LJ. J Neurosci. 1986;6:1338–1348. doi: 10.1523/JNEUROSCI.06-05-01338.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao A, Cha EM, Craig AM. J Neurosci. 2000;20:8344–8353. doi: 10.1523/JNEUROSCI.20-22-08344.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson TR, Shah PA, Benson DL. Neuropharmacology. 2004;47:694–705. doi: 10.1016/j.neuropharm.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 41.Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. Nat Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- 42.Changeux JP, Danchin A. Nature. 1976;264:705–712. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- 43.Rotzler S, Schramek H, Brenner HR. Nature. 1991;349:337–339. doi: 10.1038/349337a0. [DOI] [PubMed] [Google Scholar]
- 44.Mejat A, Ramond F, Bassel-Duby R, Khochbin S, Olson EN, Schaeffer L. Nat Neurosci. 2005;8:313–321. doi: 10.1038/nn1408. [DOI] [PubMed] [Google Scholar]
- 45.Tang J, Jo SA, Burden SJ. Development (Cambridge, UK) 1994;120:1799–1804. doi: 10.1242/dev.120.7.1799. [DOI] [PubMed] [Google Scholar]
- 46.Dichter MA, Fischbach GD. J Physiol. 1977;267:281–298. doi: 10.1113/jphysiol.1977.sp011813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmed Z, Walker PS, Fellows RE. J Neurosci. 1983;3:2448–2462. doi: 10.1523/JNEUROSCI.03-12-02448.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nerbonne JM, Gurney AM. J Neurosci. 1989;9:3272–3286. doi: 10.1523/JNEUROSCI.09-09-03272.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCobb DP, Best PM, Beam KG. J Neurosci. 1990;10:2974–2984. doi: 10.1523/JNEUROSCI.10-09-02974.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galeffi F, Sah R, Pond BB, George A, Schwartz-Bloom RD. J Neurosci. 2004;24:4478–4488. doi: 10.1523/JNEUROSCI.0755-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.