Abstract
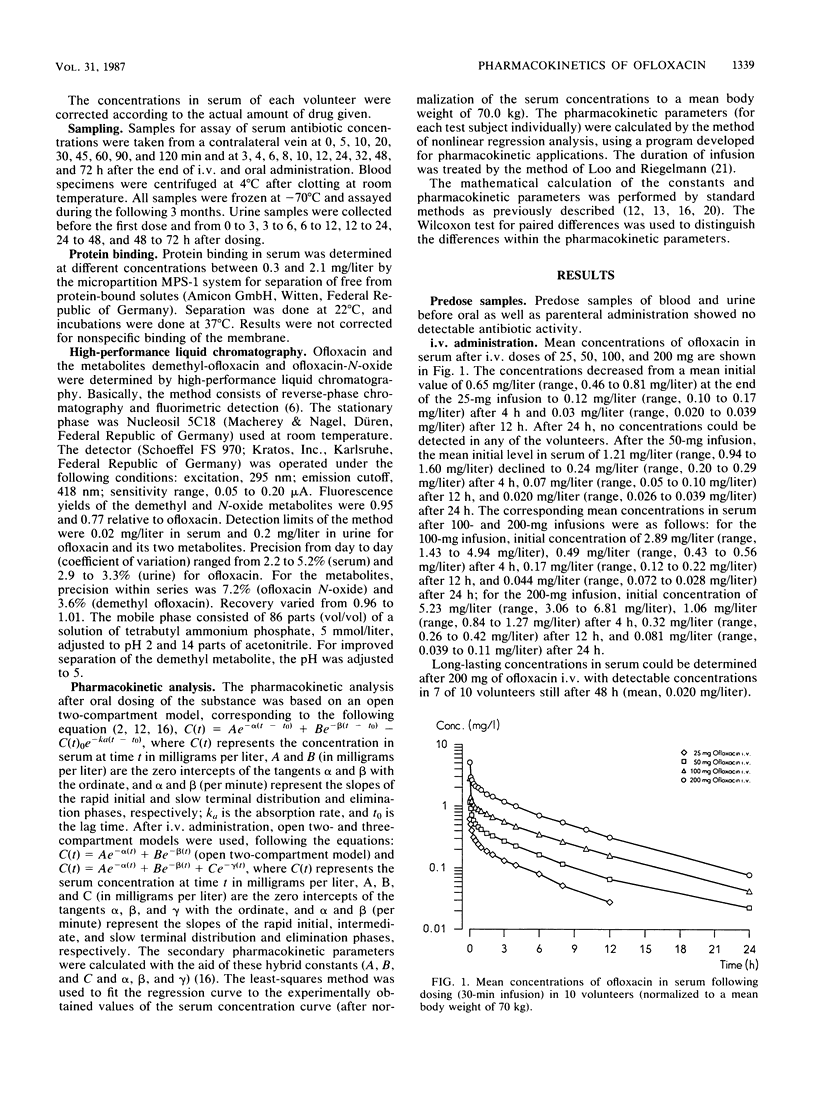
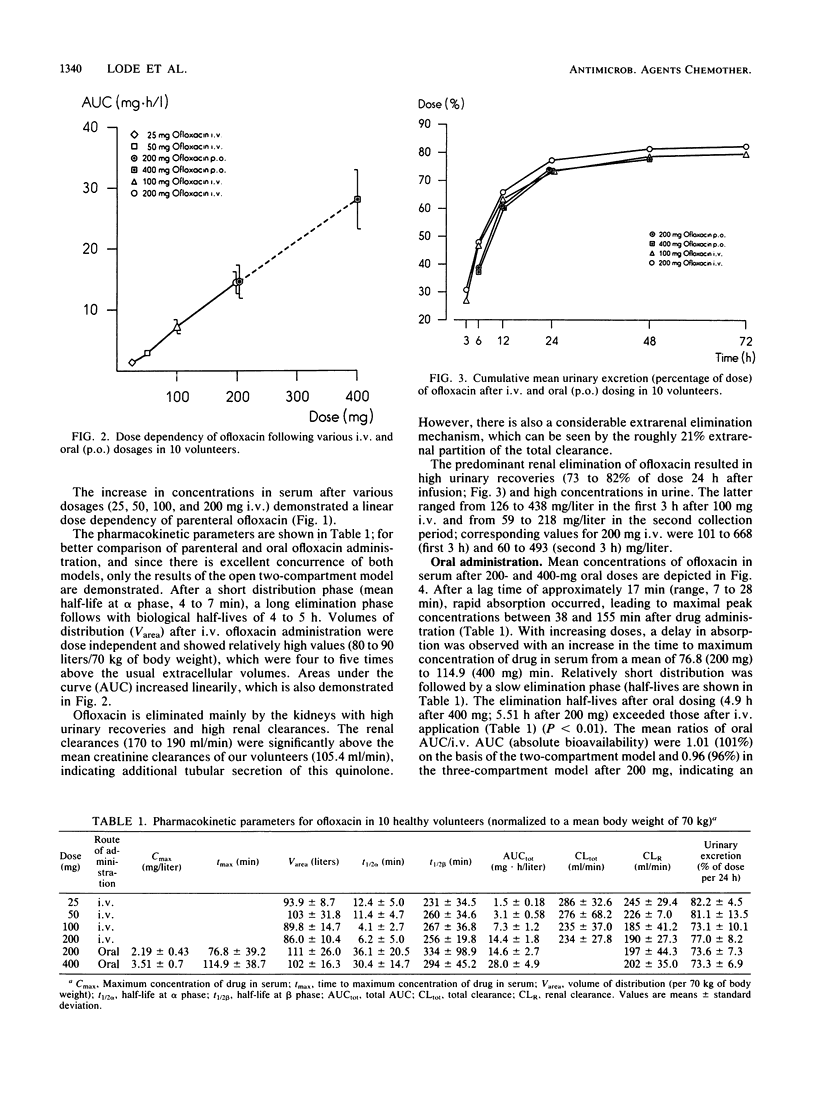
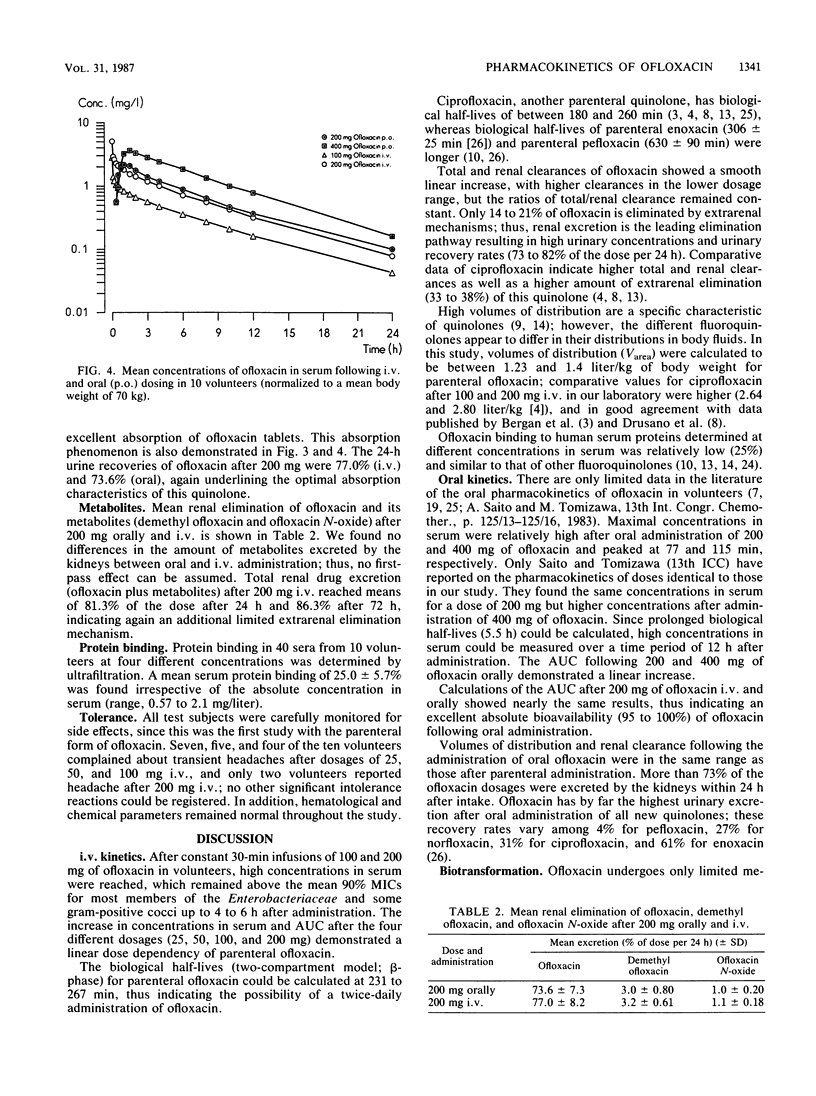
In 10 volunteers, the pharmacokinetics of ofloxacin [HOE 280, DL 8280; (+/-)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H -pyrido-[1,2,3-de] [1,4]benzoxacine-6-carboxylic acid] was determined after administration of 25, 50, 100, and 200 mg intravenously (30-min infusion) as well as 200 and 400 mg orally. Concentrations in serum and urine were measured by high-pressure liquid chromatography. Concentrations in serum following different parenteral ofloxacin dosages demonstrated dose dependency with long biological half-lives of 231 to 267 min. Pharmacokinetic parameters were calculated on the basis of open two- and three-compartment models, which yielded nearly identical results. High volumes of distribution (1.2 to 1.4 liters/kg of body weight) suggested effective diffusion into the extravascular space. High total and renal clearances indicated primarily renal excretion with additional elimination pathways, such as tubular secretion and extrarenal elimination. After oral administration, absorption was excellent, and the absolute bioavailability following 200 mg of ofloxacin could be calculated at greater than 0.95. Maximal concentrations in serum were attained 1.2 to 1.9 h after dosing; areas under the curve increased in proportion to dose between 200 and 400 mg of oral ofloxacin. The amount of known metabolites (demethyl and N-oxide compounds) excreted in urine reached only 4.3% (intravenously) and 4.0% (orally). Transient headaches in some volunteers were the only side effects registered.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. M., Heppleston C., Richmond S. J. Comparison of the in vitro activities of ofloxacin and tetracycline against Chlamydia trachomatis as assessed by indirect immunofluorescence. Antimicrob Agents Chemother. 1984 Jul;26(1):13–16. doi: 10.1128/aac.26.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benet L. Z. Effect of route of administration and distribution on drug action. J Pharmacokinet Biopharm. 1978 Dec;6(6):559–585. doi: 10.1007/BF01062110. [DOI] [PubMed] [Google Scholar]
- Bergan T., Thorsteinsson S. B., Kolstad I. M., Johnsen S. Pharmacokinetics of ciprofloxacin after intravenous and increasing oral doses. Eur J Clin Microbiol. 1986 Apr;5(2):187–192. doi: 10.1007/BF02013984. [DOI] [PubMed] [Google Scholar]
- Borner K., Höffken G., Lode H., Koeppe P., Prinzing C., Glatzel P., Wiley R., Olschewski P., Sievers B., Reinitz D. Pharmacokinetics of ciprofloxacin in healthy volunteers after oral and intravenous administration. Eur J Clin Microbiol. 1986 Apr;5(2):179–186. doi: 10.1007/BF02013983. [DOI] [PubMed] [Google Scholar]
- Drusano G. L., Plaisance K. I., Forrest A., Standiford H. C. Dose ranging study and constant infusion evaluation of ciprofloxacin. Antimicrob Agents Chemother. 1986 Sep;30(3):440–443. doi: 10.1128/aac.30.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass R. J. The quinolones. Ann Intern Med. 1985 Mar;102(3):400–402. doi: 10.7326/0003-4819-102-3-400. [DOI] [PubMed] [Google Scholar]
- Frydman A. M., Le Roux Y., Lefebvre M. A., Djebbar F., Fourtillan J. B., Gaillot J. Pharmacokinetics of pefloxacin after repeated intravenous and oral administration (400 mg bid) in young healthy volunteers. J Antimicrob Chemother. 1986 Apr;17 (Suppl B):65–79. doi: 10.1093/jac/17.suppl_b.65. [DOI] [PubMed] [Google Scholar]
- Goossens H., De Mol P., Coignau H., Levy J., Grados O., Ghysels G., Innocent H., Butzler J. P. Comparative in vitro activities of aztreonam, ciprofloxacin, norfloxacin, ofloxacin, HR 810 (a new cephalosporin), RU28965 (a new macrolide), and other agents against enteropathogens. Antimicrob Agents Chemother. 1985 Mar;27(3):388–392. doi: 10.1128/aac.27.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt D. J., Koch-Weser J. Clinical pharmacokinetics (second of two parts). N Engl J Med. 1975 Nov 6;293(19):964–970. doi: 10.1056/NEJM197511062931905. [DOI] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S. The fluoroquinolones: pharmacology, clinical uses, and toxicities in humans. Antimicrob Agents Chemother. 1985 Nov;28(5):716–721. doi: 10.1128/aac.28.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höffken G., Lode H., Prinzing C., Borner K., Koeppe P. Pharmacokinetics of ciprofloxacin after oral and parenteral administration. Antimicrob Agents Chemother. 1985 Mar;27(3):375–379. doi: 10.1128/aac.27.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmerich B., Small G. J., Pennington J. E. Comparative evaluation of ciprofloxacin, enoxacin, and ofloxacin in experimental Pseudomonas aeruginosa pneumonia. Antimicrob Agents Chemother. 1986 Mar;29(3):395–399. doi: 10.1128/aac.29.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienel G. Vergleich der Pharmakokinetik von Epicillin und Ampicillin. Arzneimittelforschung. 1976;26(5):781–789. [PubMed] [Google Scholar]
- King A., Shannon K., Phillips I. The in-vitro activities of enoxacin and ofloxacin compared with that of ciprofloxacin. J Antimicrob Chemother. 1985 May;15(5):551–558. doi: 10.1093/jac/15.5.551. [DOI] [PubMed] [Google Scholar]
- Koeppe P., Hamann C. A program for non-linear regression analysis to be used on desk-top computers. Comput Programs Biomed. 1980 Dec;12(2-3):121–128. doi: 10.1016/0010-468x(80)90058-6. [DOI] [PubMed] [Google Scholar]
- Lockley M. R., Wise R., Dent J. The pharmacokinetics and tissue penetration of ofloxacin. J Antimicrob Chemother. 1984 Dec;14(6):647–652. doi: 10.1093/jac/14.6.647. [DOI] [PubMed] [Google Scholar]
- Lode H., Elvers A., Koeppe P., Borner K. Comparative pharmacokinetics of apalcillin and piperacillin. Antimicrob Agents Chemother. 1984 Jan;25(1):105–108. doi: 10.1128/aac.25.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo J. C., Riegelman S. Assessment of pharmacokinetic constants from postinfusion blood curves obtained after I.V. infusion. J Pharm Sci. 1970 Jan;59(1):53–55. doi: 10.1002/jps.2600590107. [DOI] [PubMed] [Google Scholar]
- Seibert G., Limbert M., Klesel N. Comparison of the antibacterial in vitro and in vivo activity of ofloxacin (HOE 280 DL 8280) and nalidixic acid analogues. Eur J Clin Microbiol. 1983 Dec;2(6):548–553. doi: 10.1007/BF02016563. [DOI] [PubMed] [Google Scholar]
- Swanson B. N., Boppana V. K., Vlasses P. H., Rotmensch H. H., Ferguson R. K. Norfloxacin disposition after sequentially increasing oral doses. Antimicrob Agents Chemother. 1983 Feb;23(2):284–288. doi: 10.1128/aac.23.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Lister D., McNulty C. A., Griggs D., Andrews J. M. The comparative pharmacokinetics of five quinolones. J Antimicrob Chemother. 1986 Nov;18 (Suppl 500):71–81. doi: 10.1093/jac/18.supplement_d.71. [DOI] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrob Agents Chemother. 1985 Oct;28(4):581–586. doi: 10.1128/aac.28.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]



