Abstract
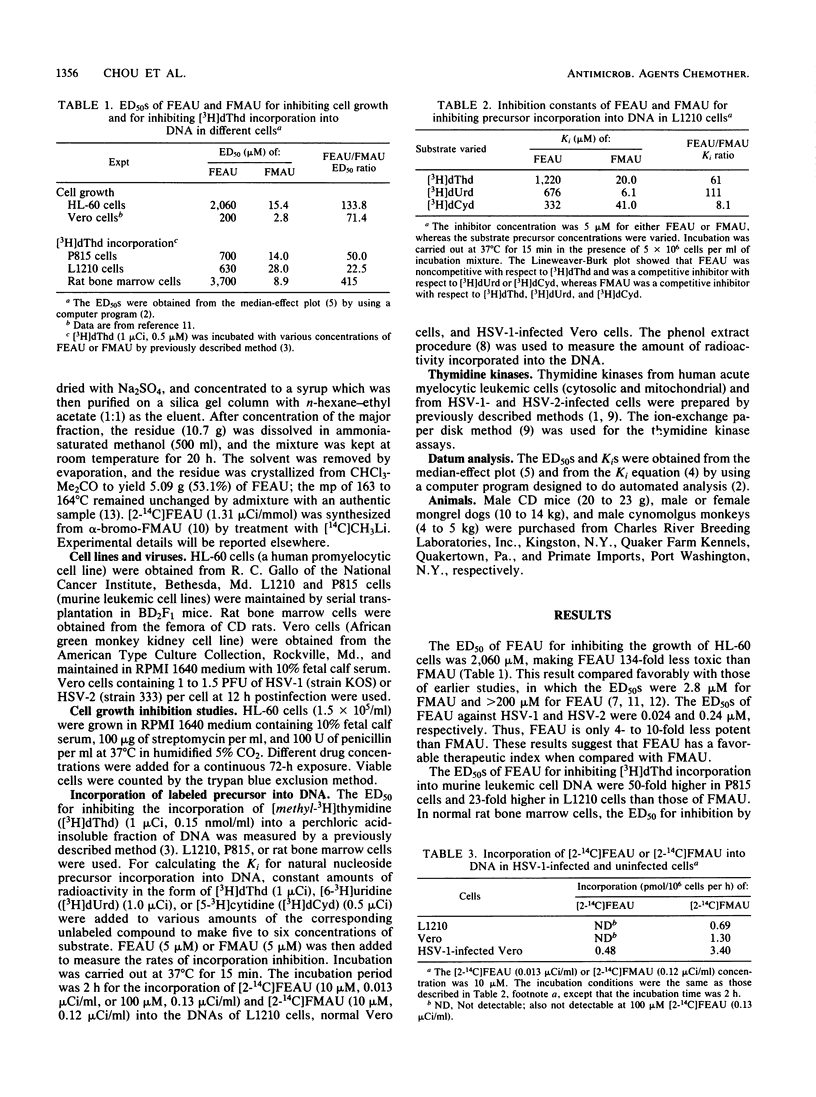
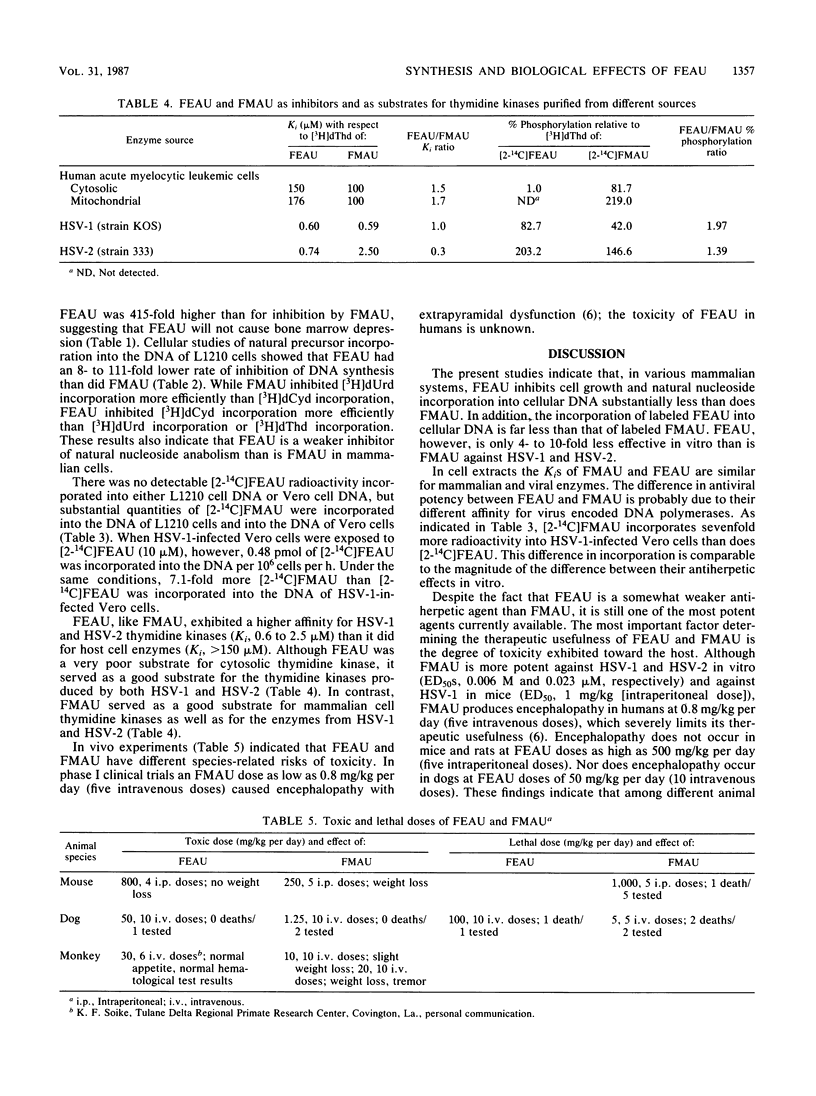
2'-Fluoro-5-ethyl-1-beta-D-arabinofuranosyluracil (FEAU) was synthesized, and its biological activities were compared with those of 2'-fluoro-5-methyl-1-beta-D-arabinofuranosyluracil (FMAU). Earlier studies indicated that both compounds showed potent anti-herpes simplex virus activity, with a 50% effective dose (ED50) of less than 0.25 microM. In the present study the cell growth inhibitory activity of FEAU (ED50, 200 to 2,060 microM) was found to be about 100-fold less than that of FMAU. With an ED50 ranging from 630 to 3,700 microM, FEAU only weakly inhibited thymidine incorporation into DNA, as compared with FMAU with an ED50 of 9 to 28 microM. Following exposure to [2-14C]FEAU (100 microM), 0.48 pmol/10(6) cells per h was incorporated into the DNA of herpes simplex virus type 1-infected Vero cells, whereas no detectable incorporation was found in uninfected Vero cells or L1210 cells. The Ki of FEAU for thymidine kinase purified from human leukemic cells was greater than 150 microM. For herpes simplex virus type 1- and 2-encoded thymidine kinases, the Kis were 0.6 and 0.74 microM, respectively. Both FEAU and FMAU were relatively nontoxic for mice, with a 50% lethal dose of greater than 800 mg/kg per day (four intraperitoneal doses). However, the lethal dose of FEAU for dogs was 100 mg/kg per day (10 intravenous doses), a dose which is 40- to 80-fold greater than the toxic dose of FMAU. These results suggest that FEAU is a worthy candidate for further development as an antiherpetic agent.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheng Y. C., Dutschman G., Fox J. J., Watanabe K. A., Machida H. Differential activity of potential antiviral nucleoside analogs on herpes simplex virus-induced and human cellular thymidine kinases. Antimicrob Agents Chemother. 1981 Sep;20(3):420–423. doi: 10.1128/aac.20.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou T. C., Burchenal J. H., Schmid F. A., Braun T. J., Su T. L., Watanabe K. A., Fox J. J., Philips F. S. Biochemical effects of 2'-fluoro-5-methyl-1-beta-D-arabinofuranosyluracil and 2'-fluoro-5-iodo-1-beta-D-arabinofuranosylcytosine in mouse leukemic cells sensitive and resistant to 1-beta-D-arabinofuranosylcytosine. Cancer Res. 1982 Oct;42(10):3957–3963. [PubMed] [Google Scholar]
- Chou T. C., Lopez C., Colacino J. M., Feinberg A., Watanabe K. A., Fox J. J., Philips F. S. Metabolic competition studies of 2'-fluoro-5-iodo-1-beta-d-arabinofuranosylcytosine in vero cells and herpes simplex type 1-infected vero cells. Mol Pharmacol. 1984 Nov;26(3):587–593. [PubMed] [Google Scholar]
- Chou T. C., Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Fanucchi M. P., Leyland-Jones B., Young C. W., Burchenal J. H., Watanabe K. A., Fox J. J. Phase I trial of 1-(2'-deoxy-2'-fluoro-1-beta-D-arabinofuranosyl)-5-methyluracil (FMAU). Cancer Treat Rep. 1985 Jan;69(1):55–59. [PubMed] [Google Scholar]
- Grant A. J., Feinberg A., Chou T. C., Watanabe K. A., Fox J. J., Philips F. S. Incorporation of metabolites of 2'-fluoro-5-iodo-1-beta-D-arabinofuranosylcytosine into deoxyribonucleic acid of neoplastic and normal mammalian tissues. Biochem Pharmacol. 1982 Mar 15;31(6):1103–1108. doi: 10.1016/0006-2952(82)90349-5. [DOI] [PubMed] [Google Scholar]
- Lee L. S., Cheng Y. C. Human deoxythymidine kinase. I. Purification and general properties of the cytoplasmic and mitochondrial isozymes derived from blast cells of acute myelocytic leukemia. J Biol Chem. 1976 May 10;251(9):2600–2604. [PubMed] [Google Scholar]
- Perlman M. E., Watanabe K. A., Schinazi R. F., Fox J. J. Nucleosides. 133. Synthesis of 5-alkenyl-1-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)cytosines and related pyrimidine nucleosides as potential antiviral agents. J Med Chem. 1985 Jun;28(6):741–748. doi: 10.1021/jm00383a009. [DOI] [PubMed] [Google Scholar]
- Schinazi R. F., Fox J. J., Watanabe K. A., Nahmias A. J. Activities of 1-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)-5-iodocytosine and its metabolites against herpes simplex virus types 1 and 2 in cell culture and in mice infected intracerebrally with herpes simplex virus type 2. Antimicrob Agents Chemother. 1986 Jan;29(1):77–84. doi: 10.1128/aac.29.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K. A., Su T. L., Reichman U., Greenberg N., Lopez C., Fox J. J. Nucleosides. 129. Synthesis of antiviral nucleosides: 5-alkenyl-1-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)uracils. J Med Chem. 1984 Jan;27(1):91–94. doi: 10.1021/jm00367a020. [DOI] [PubMed] [Google Scholar]



