Abstract
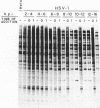
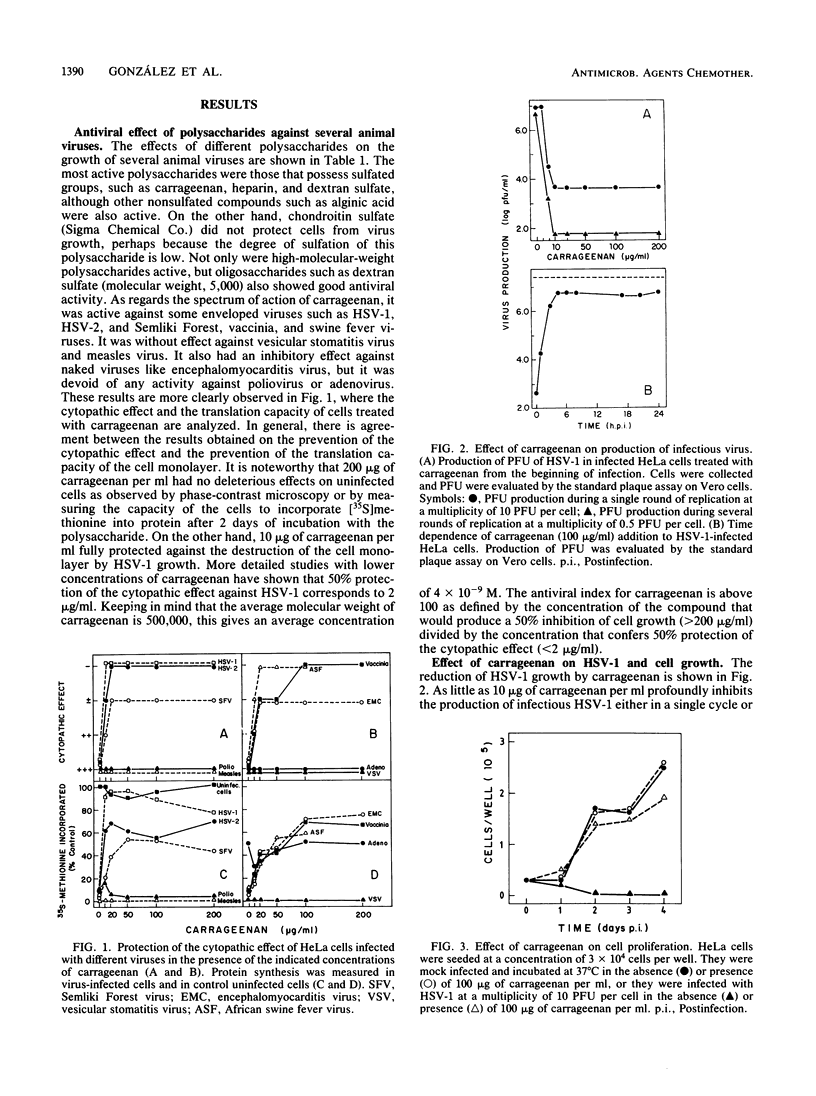
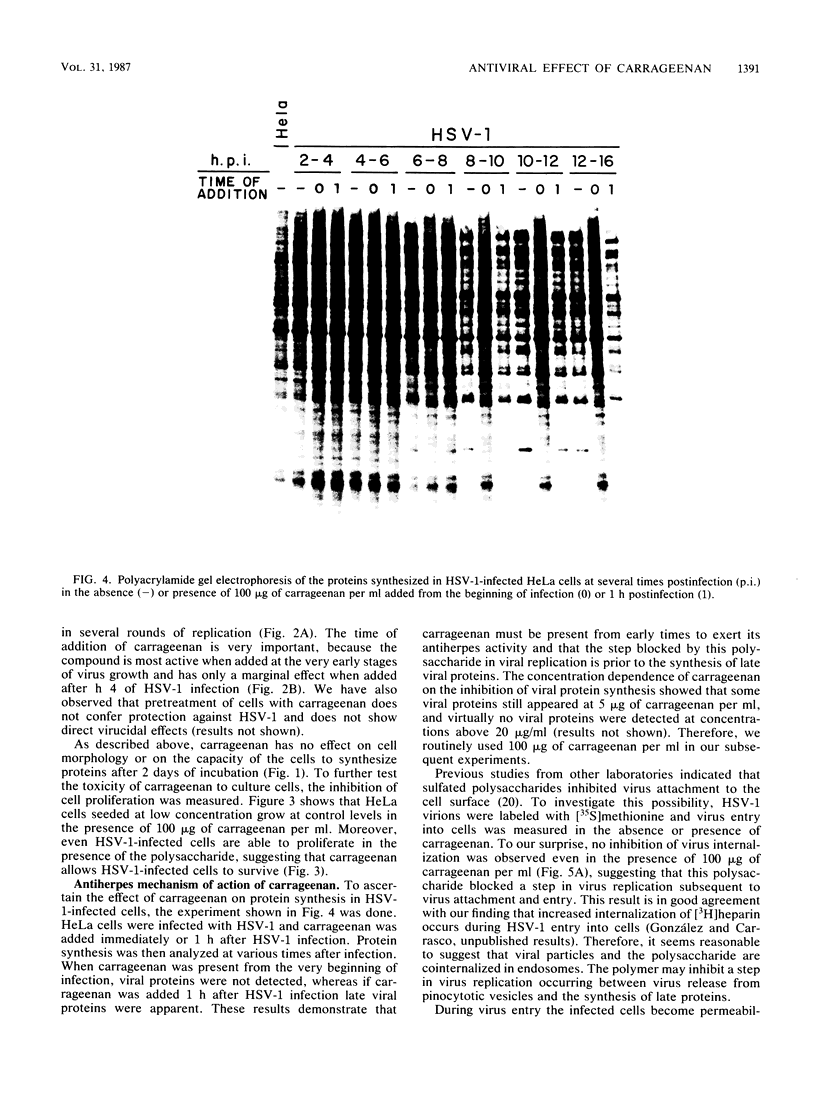
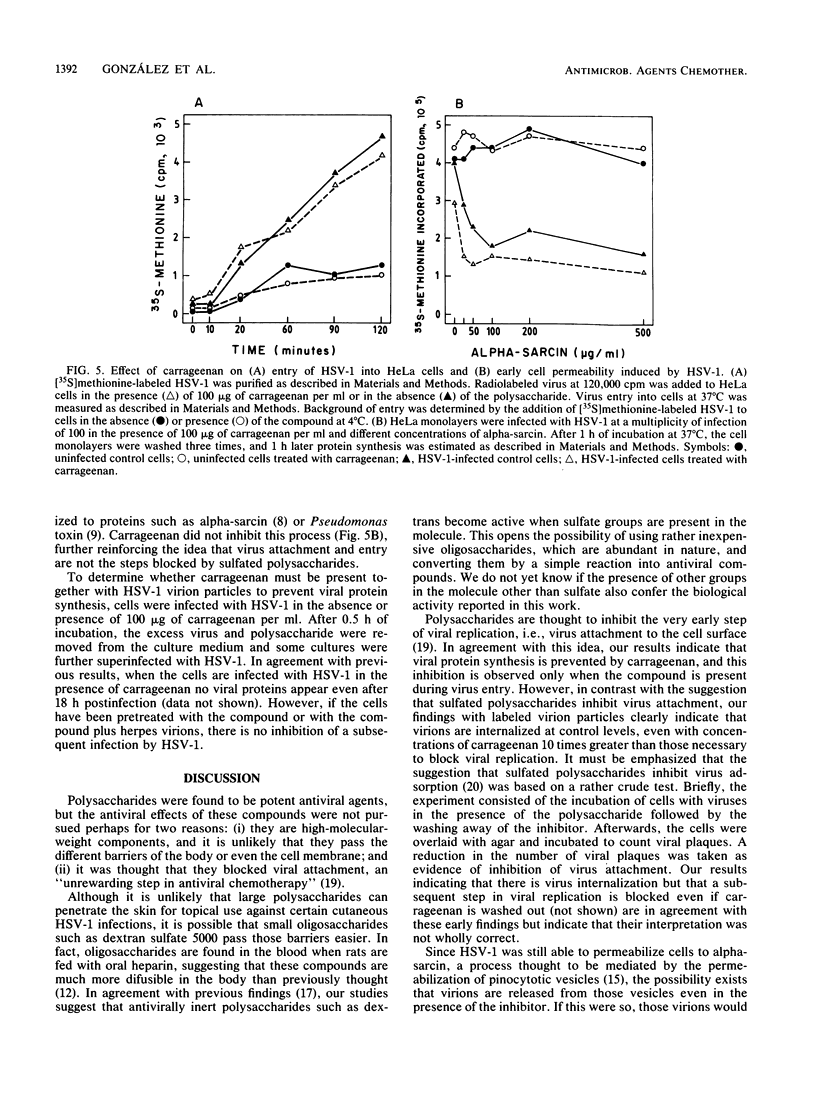
A number of polysaccharides showed good antiviral activity against several animal viruses. At 5 micrograms/ml, carrageenan prevented the cell monolayer from destruction by herpes simplex virus type 1 (HSV-1) growth. At 10 micrograms/ml, carrageenan reduced the formation of new infectious HSV-1 by almost five logs. No cytotoxic effects were detected with concentrations of carrageenan up to 200 micrograms/ml. When 10 micrograms of carrageenan per ml was added at the beginning of HSV-1 infection of HeLa cells, there was potent inhibition of viral protein synthesis, and the cells continued synthesizing cellular proteins. This did not occur if carrageenan was added 1 h after HSV-1 infection. The use of [35S]methionine-labeled virions to analyze the entry of HSV-1 or Semliki Forest virions into cells indicated that carrageenan had no effect on virus attachment or virus entry. Moreover, carrageenan did not block the early permeabilization of cells to the toxic protein alpha-sarcin. These results suggest that this sulfated polysaccharide inhibits a step in virus replication subsequent to viral internalization but prior to the onset of late viral protein synthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alarcón B., Lacal J. C., Fernández-Sousa J. M., Carrasco L. Screening for new compounds with antiherpes activity. Antiviral Res. 1984 Oct;4(5):231–244. doi: 10.1016/0166-3542(84)90029-9. [DOI] [PubMed] [Google Scholar]
- CHOPPIN P. W., EGGERS H. J. Heterogeneity of Coxsackie B4 virus: two kinds of particles which differ in antibody sensitivity, growth rate, and plaque size. Virology. 1962 Nov;18:470–476. doi: 10.1016/0042-6822(62)90037-5. [DOI] [PubMed] [Google Scholar]
- COLTER J. S., DAVIES M. A., CAMPBELL J. B. STUDIES OF THREE VARIANTS OF MENGO ENCEPHALOMYELITIS VIRUS. II. INHIBITION OF INTERACTION WITH L CELLS BY AN AGAR INHIBITOR AND BY PROTAMINE. Virology. 1964 Dec;24:578–585. doi: 10.1016/0042-6822(64)90210-7. [DOI] [PubMed] [Google Scholar]
- De Somer P., De Clercq E., Billiau A., Schonne E., Claesen M. Antiviral activity of polyacrylic and polymethacrylic acids. II. Mode of action in vivo. J Virol. 1968 Sep;2(9):886–893. doi: 10.1128/jvi.2.9.886-893.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar C. F., Dickinson A. G. Prolongation of scrapie incubation period by an injection of dextran sulphate 500 within the month before or after infection. J Gen Virol. 1986 Mar;67(Pt 3):463–473. doi: 10.1099/0022-1317-67-3-463. [DOI] [PubMed] [Google Scholar]
- Fernández-Puentes C., Carrasco L. Viral infection permeabilizes mammalian cells to protein toxins. Cell. 1980 Jul;20(3):769–775. doi: 10.1016/0092-8674(80)90323-2. [DOI] [PubMed] [Google Scholar]
- FitzGerald D. J., Padmanabhan R., Pastan I., Willingham M. C. Adenovirus-induced release of epidermal growth factor and pseudomonas toxin into the cytosol of KB cells during receptor-mediated endocytosis. Cell. 1983 Feb;32(2):607–617. doi: 10.1016/0092-8674(83)90480-4. [DOI] [PubMed] [Google Scholar]
- Folkman J., Langer R., Linhardt R. J., Haudenschild C., Taylor S. Angiogenesis inhibition and tumor regression caused by heparin or a heparin fragment in the presence of cortisone. Science. 1983 Aug 19;221(4612):719–725. doi: 10.1126/science.6192498. [DOI] [PubMed] [Google Scholar]
- Hochberg E., Becker Y. Adsorption, penetration and uncoating of herpes simplex virus. J Gen Virol. 1968 Mar;2(2):231–241. doi: 10.1099/0022-1317-2-2-231. [DOI] [PubMed] [Google Scholar]
- LIEBHABER H., TAKEMOTO K. K. THE BASIS FOR THE SIZE DIFFERENCES IN PLAQUES PRODUCED BY VARIANTS OF ENCEPHALOMYOCARDITIS (EMC) VIRUS. Virology. 1963 Aug;20:559–566. doi: 10.1016/0042-6822(63)90280-0. [DOI] [PubMed] [Google Scholar]
- Larsen A. K., Lund D. P., Langer R., Folkman J. Oral heparin results in the appearance of heparin fragments in the plasma of rats. Proc Natl Acad Sci U S A. 1986 May;83(9):2964–2968. doi: 10.1073/pnas.83.9.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan T. C., Finkelstein M. S. Interferon-stimulating and in vivo antiviral effects of various synthetic anionic polymers. Virology. 1968 Jul;35(3):363–374. doi: 10.1016/0042-6822(68)90215-8. [DOI] [PubMed] [Google Scholar]
- Muñoz A., Castrillo J. L., Carrasco L. Modification of membrane permeability during Semliki Forest virus infection. Virology. 1985 Oct 30;146(2):203–212. doi: 10.1016/0042-6822(85)90004-2. [DOI] [PubMed] [Google Scholar]
- NAHMIAS A. J., KIBRICK S., BERNFELD P. EFFECT OF SYNTHETIC AND BIOLOGICAL POLYANIONS ON HERPES SIMPLEX VIRUS. Proc Soc Exp Biol Med. 1964 Apr;115:993–996. doi: 10.3181/00379727-115-29098. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Kibrick S. Inhibitory effect of heparin on herpes simplex virus. J Bacteriol. 1964 May;87(5):1060–1066. doi: 10.1128/jb.87.5.1060-1066.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. A. Structure, conformation, and mechanism in the formation of polysaccharide gels and networks. Adv Carbohydr Chem Biochem. 1969;24:267–332. doi: 10.1016/s0065-2318(08)60352-2. [DOI] [PubMed] [Google Scholar]
- TAKEMOTO K. K., FABISCH P. INHIBITION OF HERPES VIRUS BY NATURAL AND SYNTHETIC ACID POLYSACCHARIDES. Proc Soc Exp Biol Med. 1964 May;116:140–144. doi: 10.3181/00379727-116-29183. [DOI] [PubMed] [Google Scholar]
- TAKEMOTO K. K., LIEBHABER H. Virus-polysaccharide interactions. I. An agar polysaccharide determining plaque morphology of EMC virus. Virology. 1961 Aug;14:456–462. doi: 10.1016/0042-6822(61)90338-5. [DOI] [PubMed] [Google Scholar]
- TAKEMOTO K. K., LIEBHABER H. Virus-polysaccharide interactions. II. Enhancement of plague formation and the detection of variants of poliovirus with dextran sulfate. Virology. 1962 Jul;17:499–501. doi: 10.1016/0042-6822(62)90148-4. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K. Plaque mutants of animal viruses. Prog Med Virol. 1966;8:314–348. [PubMed] [Google Scholar]
- VAHERI A., CANTELL K. THE EFFECT OF HEPARIN ON HERPES SIMPLEX VIRUS. Virology. 1963 Dec;21:661–662. doi: 10.1016/0042-6822(63)90242-3. [DOI] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L. Mechanism of enhancement of virus plaques by cationic polymers. J Virol. 1968 Apr;2(4):267–274. doi: 10.1128/jvi.2.4.267-274.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Clercq E., Luczak M. Antiviral activity of carbopol, a cross-linked polycarboxylate. Arch Virol. 1976;52(1-2):151–158. doi: 10.1007/BF01317874. [DOI] [PubMed] [Google Scholar]