Abstract
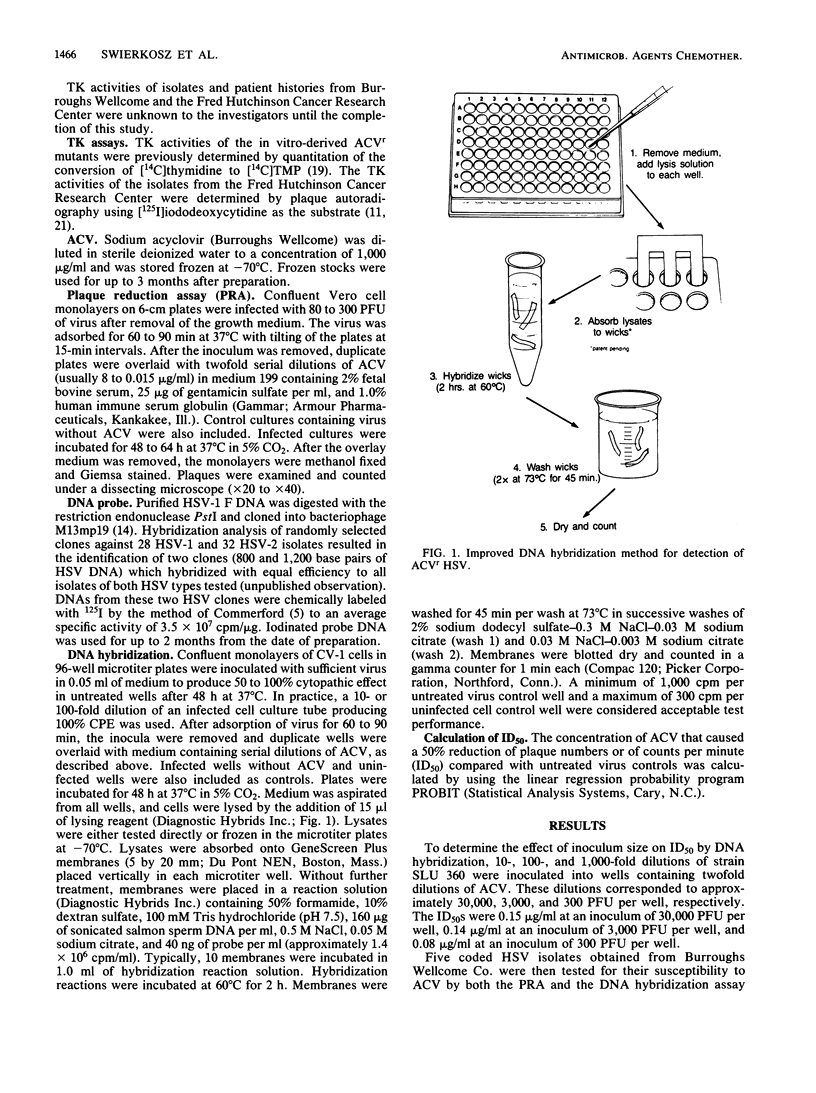
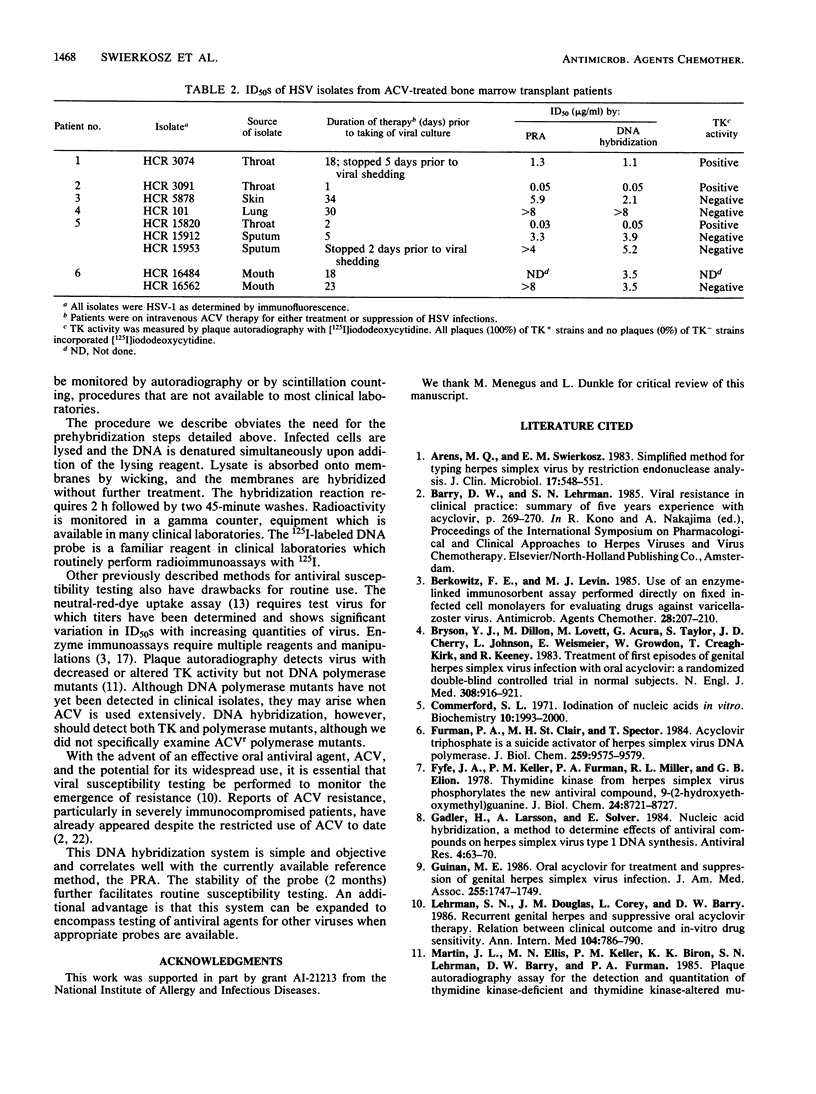
A simplified DNA hybridization method was developed to detect acyclovir-resistant isolates of herpes simplex virus. Herpes simplex virus-infected cell cultures in microtiter plates were treated with concentrations of acyclovir ranging from 8 to 0.015 micrograms/ml. At 48 h postinfection, infected cells were lysed by a one-step procedure and lysates were absorbed to membranes. Without further treatment, membranes were hybridized by using a herpes simplex virus-specific radioiodinated probe. The membranes were then washed and counted in a gamma counter. The elapsed time for assay performance was 4 h. Parallel plaque reduction assays were performed for comparison. The mean 50% inhibitory dose of in vivo- and in vitro-derived acyclovir-resistant, thymidine kinase-negative isolates was greater than 2 micrograms/ml by DNA hybridization. The 50% inhibitory dose of acyclovir-susceptible, thymidine kinase-positive isolates ranged from 0.01 to 1.1 micrograms/ml. This assay is simple and objective and should facilitate antiviral susceptibility testing in diagnostic laboratories.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arens M. Q., Swierkosz E. M. Simplified method for typing herpes simplex virus by restriction endonuclease analysis. J Clin Microbiol. 1983 Mar;17(3):548–551. doi: 10.1128/jcm.17.3.548-551.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz F. E., Levin M. J. Use of an enzyme-linked immunosorbent assay performed directly on fixed infected cell monolayers for evaluating drugs against varicella-zoster virus. Antimicrob Agents Chemother. 1985 Aug;28(2):207–210. doi: 10.1128/aac.28.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson Y. J., Dillon M., Lovett M., Acuna G., Taylor S., Cherry J. D., Johnson B. L., Wiesmeier E., Growdon W., Creagh-Kirk T. Treatment of first episodes of genital herpes simplex virus infection with oral acyclovir. A randomized double-blind controlled trial in normal subjects. N Engl J Med. 1983 Apr 21;308(16):916–921. doi: 10.1056/NEJM198304213081602. [DOI] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Furman P. A., St Clair M. H., Spector T. Acyclovir triphosphate is a suicide inactivator of the herpes simplex virus DNA polymerase. J Biol Chem. 1984 Aug 10;259(15):9575–9579. [PubMed] [Google Scholar]
- Fyfe J. A., Keller P. M., Furman P. A., Miller R. L., Elion G. B. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J Biol Chem. 1978 Dec 25;253(24):8721–8727. [PubMed] [Google Scholar]
- Gadler H., Larsson A., Sølver E. Nucleic acid hybridization, a method to determine effects of antiviral compounds on herpes simplex virus type 1 DNA synthesis. Antiviral Res. 1984 Apr;4(1-2):63–70. doi: 10.1016/0166-3542(84)90026-3. [DOI] [PubMed] [Google Scholar]
- Guinan M. E. Oral acyclovir for treatment and suppression of genital herpes simplex virus infection. A review. JAMA. 1986 Apr 4;255(13):1747–1749. [PubMed] [Google Scholar]
- Lehrman S. N., Douglas J. M., Corey L., Barry D. W. Recurrent genital herpes and suppressive oral acyclovir therapy. Relation between clinical outcome and in-vitro drug sensitivity. Ann Intern Med. 1986 Jun;104(6):786–790. doi: 10.7326/0003-4819-104-6-786. [DOI] [PubMed] [Google Scholar]
- McGuirt P. V., Shaw J. E., Elion G. B., Furman P. A. Identification of small DNA fragments synthesized in herpes simplex virus-infected cells in the presence of acyclovir. Antimicrob Agents Chemother. 1984 Apr;25(4):507–509. doi: 10.1128/aac.25.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren C., Ellis M. N., Hunter G. A. A colorimetric assay for the measurement of the sensitivity of herpes simplex viruses to antiviral agents. Antiviral Res. 1983 Nov;3(4):223–234. doi: 10.1016/0166-3542(83)90001-3. [DOI] [PubMed] [Google Scholar]
- Messing J., Gronenborn B., Müller-Hill B., Hans Hopschneider P. Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. D., Wade J. C., Mitchell C. D., Saral R., Lietman P. S., Durack D. T., Levin M. J., Segreti A. C., Balfour H. H., Jr Multicenter collaborative trial of intravenous acyclovir for treatment of mucocutaneous herpes simplex virus infection in the immunocompromised host. Am J Med. 1982 Jul 20;73(1A):229–235. doi: 10.1016/0002-9343(82)90097-3. [DOI] [PubMed] [Google Scholar]
- Miller W. H., Miller R. L. Phosphorylation of acyclovir diphosphate by cellular enzymes. Biochem Pharmacol. 1982 Dec 1;31(23):3879–3884. doi: 10.1016/0006-2952(82)90305-7. [DOI] [PubMed] [Google Scholar]
- Rabalais G. P., Levin M. J., Berkowitz F. E. Rapid herpes simplex virus susceptibility testing using an enzyme-linked immunosorbent assay performed in situ on fixed virus-infected monolayers. Antimicrob Agents Chemother. 1987 Jun;31(6):946–948. doi: 10.1128/aac.31.6.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibrack C. D., Gutman L. T., Wilfert C. M., McLaren C., St Clair M. H., Keller P. M., Barry D. W. Pathogenicity of acyclovir-resistant herpes simplex virus type 1 from an immunodeficient child. J Infect Dis. 1982 Nov;146(5):673–682. doi: 10.1093/infdis/146.5.673. [DOI] [PubMed] [Google Scholar]
- Swierkosz E. M., Arens M. Q., Rivetna K. A. Problems associated with the use of (E)-5-(2-bromovinyl)-2'-deoxyuridine for typing herpes simplex virus. J Clin Microbiol. 1985 Mar;21(3):459–461. doi: 10.1128/jcm.21.3.459-461.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swierkosz E. M., Arens M. Q., Schmidt R. R., Armstrong T. Evaluation of two immunofluorescence assays with monoclonal antibodies for typing of herpes simplex virus. J Clin Microbiol. 1985 Apr;21(4):643–644. doi: 10.1128/jcm.21.4.643-644.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenser R. B., Jones J. C., Ressel S. J., Fralish F. A. Thymidine plaque autoradiography of thymidine kinase-positive and thymidine kinase-negative herpesviruses. J Clin Microbiol. 1983 Jan;17(1):122–127. doi: 10.1128/jcm.17.1.122-127.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J. C., McLaren C., Meyers J. D. Frequency and significance of acyclovir-resistant herpes simplex virus isolated from marrow transplant patients receiving multiple courses of treatment with acyclovir. J Infect Dis. 1983 Dec;148(6):1077–1082. doi: 10.1093/infdis/148.6.1077. [DOI] [PubMed] [Google Scholar]
- Whitley R. J., Alford C. A., Hirsch M. S., Schooley R. T., Luby J. P., Aoki F. Y., Hanley D., Nahmias A. J., Soong S. J. Vidarabine versus acyclovir therapy in herpes simplex encephalitis. N Engl J Med. 1986 Jan 16;314(3):144–149. doi: 10.1056/NEJM198601163140303. [DOI] [PubMed] [Google Scholar]
- Whitley R. J. Interim summary of mortality in herpes simplex encephalitis and neonatal herpes simplex virus infections: vidarabine versus acyclovir. J Antimicrob Chemother. 1983 Sep;12 (Suppl B):105–112. doi: 10.1093/jac/12.suppl_b.105. [DOI] [PubMed] [Google Scholar]



