Abstract
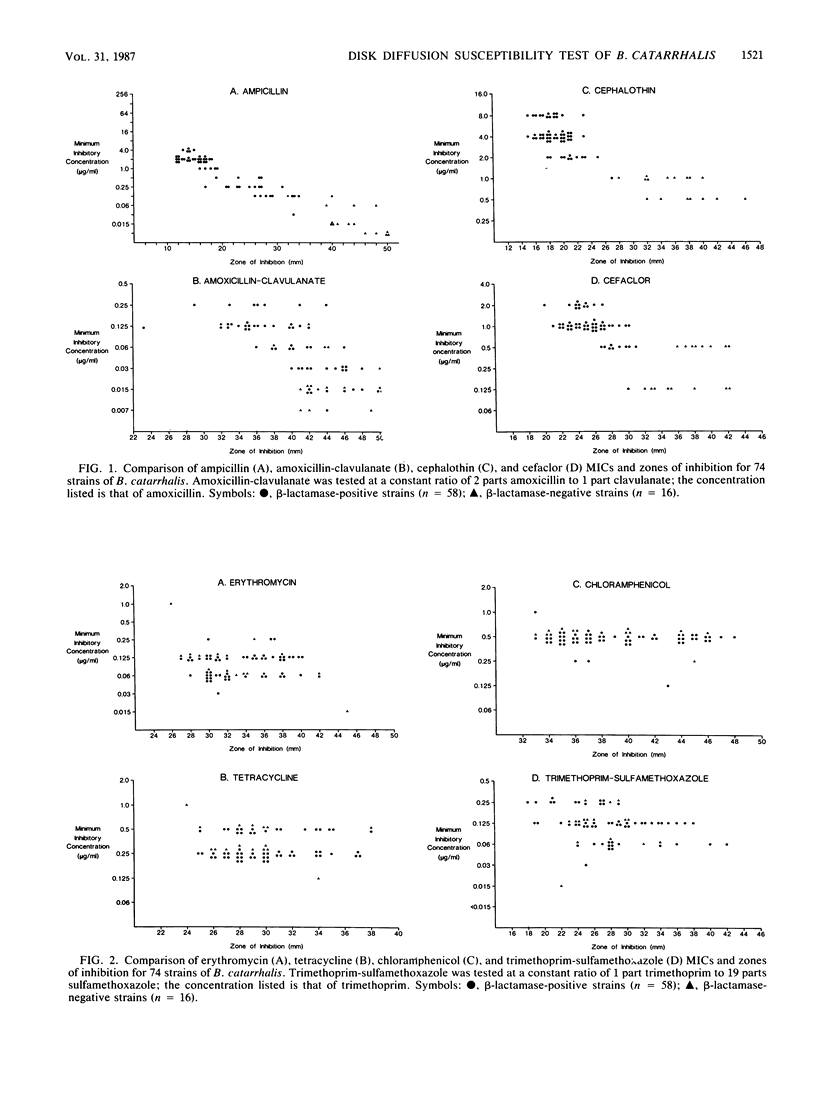
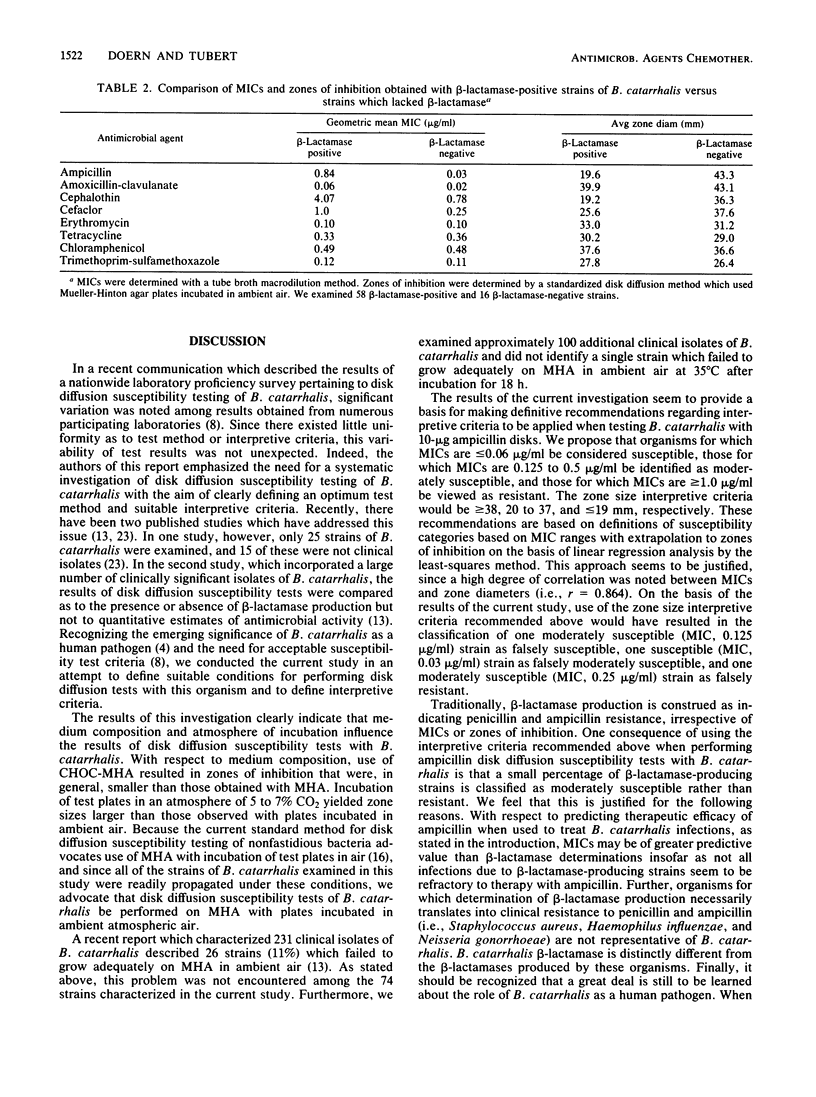
A total of 74 clinical isolates of Branhamella catarrhalis were characterized with respect to their ampicillin, amoxicillin-clavulanate, cephalothin, cefaclor, erythromycin, tetracycline, chloramphenicol, and trimethoprim-sulfamethoxazole MICs and zones of inhibition. Disk diffusion tests were performed according to the guidelines of the National Committee for Clinical Laboratory Standards with two different media (Mueller-Hinton agar and chocolate Mueller-Hinton agar) and plates incubated under two atmospheric conditions (ambient air and 5 to 7% CO2). Optimum disk diffusion test results were obtained with Mueller-Hinton agar plates incubated in ambient air with all eight antimicrobial agents. On the basis of comparisons of MICs versus zones of inhibition, the following zone diameter interpretive criteria were defined for testing B. catarrhalis with disks containing 10 micrograms of ampicillin: greater than or equal to 38 mm, susceptible; 20 to 37 mm, moderately susceptible; less than or equal to 19 mm, resistant. The respective MIC correlates were less than or equal to 0.06, 0.125 to 0.5, and greater than or equal to 1.0 micrograms/ml. Because of the absence of frankly resistant test organisms, it was not possible to make definitive recommendations pertaining to disk diffusion tests with amoxicillin-clavulanate, cephalothin, cefaclor, erythromycin, tetracycline, chloramphenicol, and trimethoprim-sulfamethoxazole. Evidence is presented, however, which suggests that the current National Committee for Clinical Laboratory Standards disk diffusion interpretive criteria for nonfastidious bacteria can be applied to B. catarrhalis, at least as they pertain to the susceptible category with cephalothin, amoxicillin-clavulanate, erythromycin, tetracycline, chloramphenicol, and trimethoprim-sulfamethoxazole. With cefaclor, a zone diameter of greater than or equal to 21 mm was determined to adequately define the susceptible category.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez S., Jones M., Holtsclaw-Berk S., Guarderas J., Berk S. L. In vitro susceptibilities and beta-lactamase production of 53 clinical isolates of Branhamella catarrhalis. Antimicrob Agents Chemother. 1985 Apr;27(4):646–647. doi: 10.1128/aac.27.4.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branhamella catarrhalis. Lancet. 1982 May 29;1(8283):1244–1245. [PubMed] [Google Scholar]
- Calder M. A., Croughan M. J., McLeod D. T., Ahmad F. The incidence and antibiotic susceptibility of Branhamella catarrhalis in respiratory infections. Drugs. 1986;31 (Suppl 3):11–16. doi: 10.2165/00003495-198600313-00005. [DOI] [PubMed] [Google Scholar]
- Davies B. I., Maesen F. P. Epidemiological and bacteriological findings on Branhamella catarrhalis respiratory infections in The Netherlands. Drugs. 1986;31 (Suppl 3):28–33. doi: 10.2165/00003495-198600313-00008. [DOI] [PubMed] [Google Scholar]
- Doern G. V. Branhamella catarrhalis--an emerging human pathogen. Diagn Microbiol Infect Dis. 1986 Mar;4(3):191–201. doi: 10.1016/0732-8893(86)90098-2. [DOI] [PubMed] [Google Scholar]
- Doern G. V., Miller M. J., Winn R. E. Branhamella (Neisseria) catarrhalis systemic disease in humans. Case reports and review of the literature. Arch Intern Med. 1981 Nov;141(12):1690–1692. [PubMed] [Google Scholar]
- Doern G. V., Siebers K. G., Hallick L. M., Morse S. A. Antibiotic susceptibility of beta-lactamase-producing strains of Branhamella (Neisseria) catarrhalis. Antimicrob Agents Chemother. 1980 Jan;17(1):24–29. doi: 10.1128/aac.17.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. A., Drew W. L., Roberts M. Branhamella (Neisseria) catarrhalis--a lower respiratory tract pathogen? J Clin Microbiol. 1981 Jun;13(6):1066–1069. doi: 10.1128/jcm.13.6.1066-1069.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Sommers H. M. Identification and antimicrobial susceptibility testing of Branhamella catarrhalis in United States laboratories, 1983-1985. Drugs. 1986;31 (Suppl 3):34–37. doi: 10.2165/00003495-198600313-00009. [DOI] [PubMed] [Google Scholar]
- Kallings I., Bengtsson S., Christensen P., Holm S. E., Lind L., Kalin M. Antibiotic sensitivity of Haemophilus influenzae, Streptococcus pneumoniae, Streptococcus pyogenes and Branhamella catarrhalis isolated from upper respiratory tract infections in Sweden. Scand J Infect Dis Suppl. 1983;39:100–105. [PubMed] [Google Scholar]
- Kallings I. Sensitivity of Branhamella catarrhalis to oral antibiotics. Drugs. 1986;31 (Suppl 3):17–22. doi: 10.2165/00003495-198600313-00006. [DOI] [PubMed] [Google Scholar]
- Kamme C. Evaluation of the in vitro sensitivity of Neisseria catarrhalis to antibiotics with respect to acute otitis media. Scand J Infect Dis. 1970;2(2):117–120. doi: 10.3109/inf.1970.2.issue-2.07. [DOI] [PubMed] [Google Scholar]
- Louie M. H., Gabay E. L., Mathisen G. E., Finegold S. M. Branhamella catarrhalis pneumonia. West J Med. 1983 Jan;138(1):47–49. [PMC free article] [PubMed] [Google Scholar]
- Luman I., Wilson R. W., Wallace R. J., Jr, Nash D. R. Disk diffusion susceptibility of Branhamella catarrhalis and relationship of beta-lactam zone size to beta-lactamase production. Antimicrob Agents Chemother. 1986 Nov;30(5):774–776. doi: 10.1128/aac.30.5.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maesen F. P., Davies B. I. Branhamella catarrhalis respiratory infections in The Netherlands. Drugs. 1986;31 (Suppl 3):83–86. doi: 10.2165/00003495-198600313-00018. [DOI] [PubMed] [Google Scholar]
- McLeod D. T., Ahmad F., Power J. T., Calder M. A., Seaton A. Bronchopulmonary infection due to Branhamella catarrhalis. Br Med J (Clin Res Ed) 1983 Nov 12;287(6403):1446–1447. doi: 10.1136/bmj.287.6403.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninane G., Joly J., Kraytman M., Piot P. Bronchopulmonary infection due to beta-lactamase-producing Branhamella catarrhalis treated with amoxycillin/clavulanic-acid. Lancet. 1978 Jul 29;2(8083):257–257. doi: 10.1016/s0140-6736(78)91763-4. [DOI] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhofer T. R., Towle D. W. Evaluation of the rapid penicillinase paper strip test for detection of beta-lactamase. J Clin Microbiol. 1982 Feb;15(2):196–199. doi: 10.1128/jcm.15.2.196-199.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A., Yamaguchi K., Shigeno Y., Kohno S., Shigeno H., Kusano N., Dotsu Y., Hara K. Clinical and bacteriological evaluation of Branhamella catarrhalis in respiratory infections. Drugs. 1986;31 (Suppl 3):87–92. doi: 10.2165/00003495-198600313-00019. [DOI] [PubMed] [Google Scholar]
- Slevin N. J., Aitken J., Thornley P. E. Clinical and microbiological features of Branhamella catarrhalis bronchopulmonary infections. Lancet. 1984 Apr 7;1(8380):782–783. doi: 10.1016/s0140-6736(84)91288-1. [DOI] [PubMed] [Google Scholar]
- Stobberingh E. E., van Eck H. J., Houben A. W., van Boven C. P. Analysis of the relationship between ampicillin resistance and beta-lactamase production in Branhamella catarrhalis. Drugs. 1986;31 (Suppl 3):23–27. doi: 10.2165/00003495-198600313-00007. [DOI] [PubMed] [Google Scholar]
- Sweeney K. G., Verghese A., Needham C. A. In vitro susceptibilities of isolates from patients with Branhamella catarrhalis pneumonia compared with those of colonizing strains. Antimicrob Agents Chemother. 1985 Apr;27(4):499–502. doi: 10.1128/aac.27.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]



