Abstract
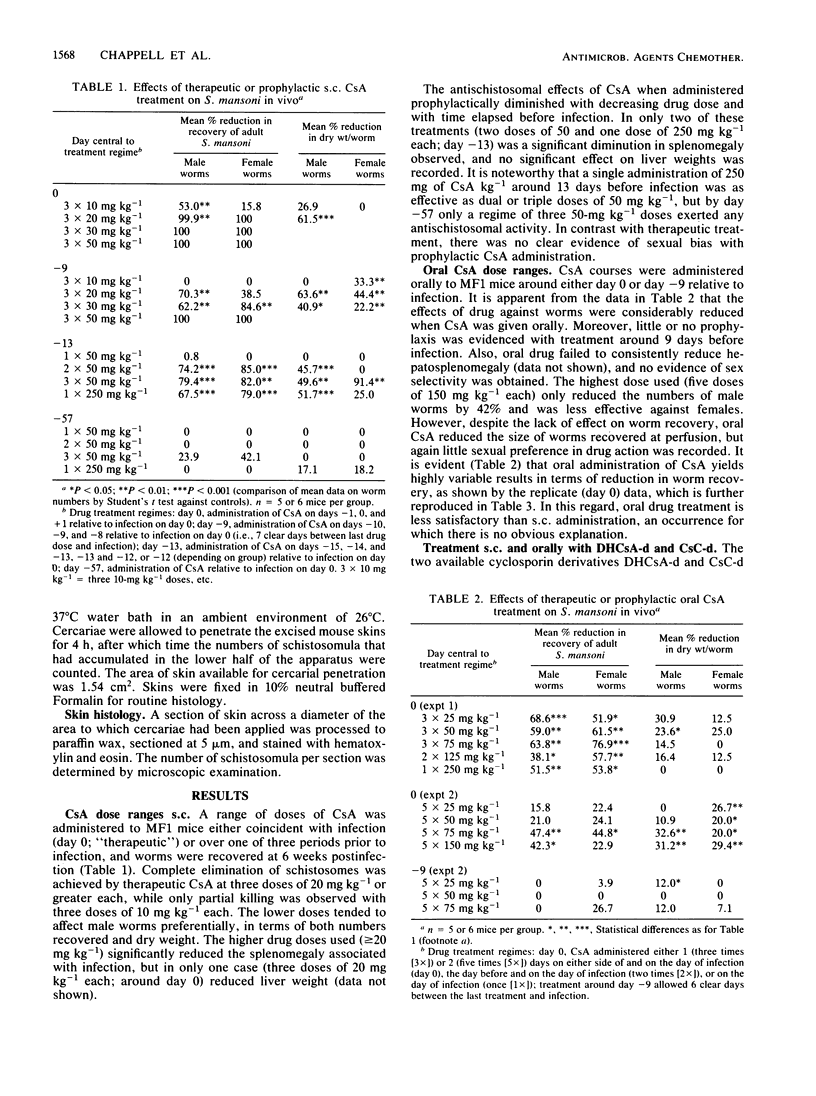
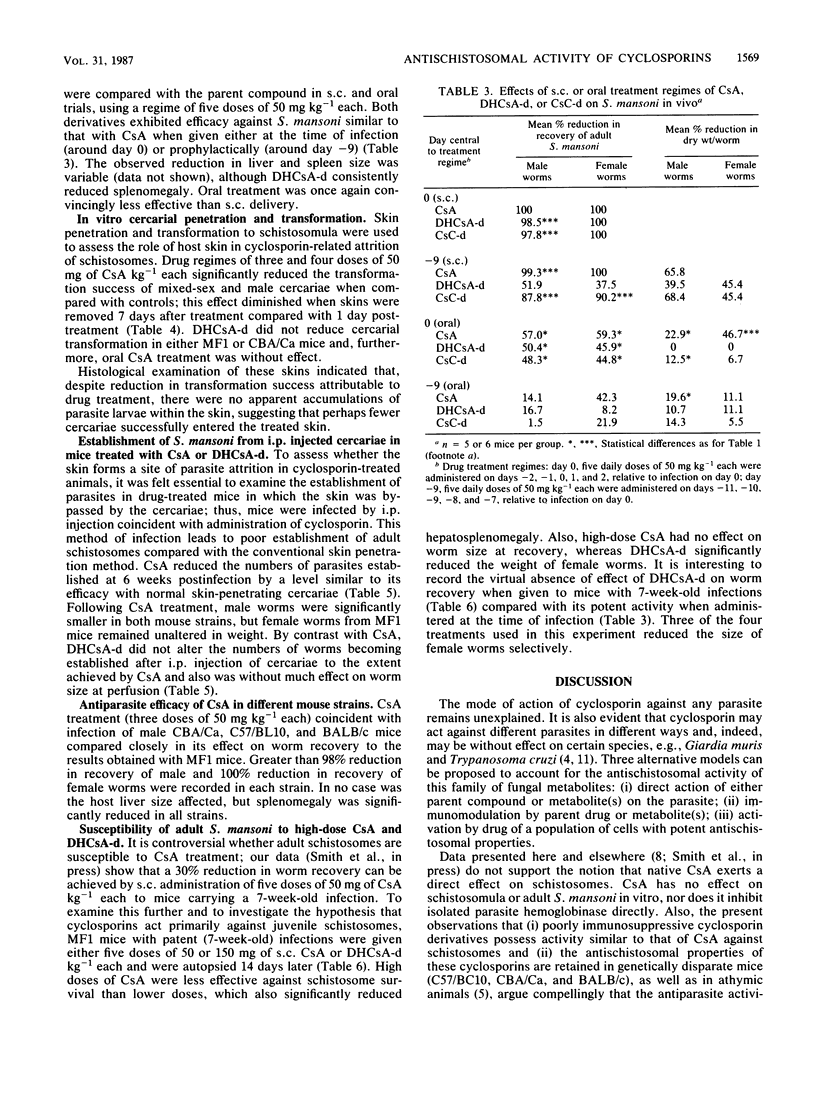
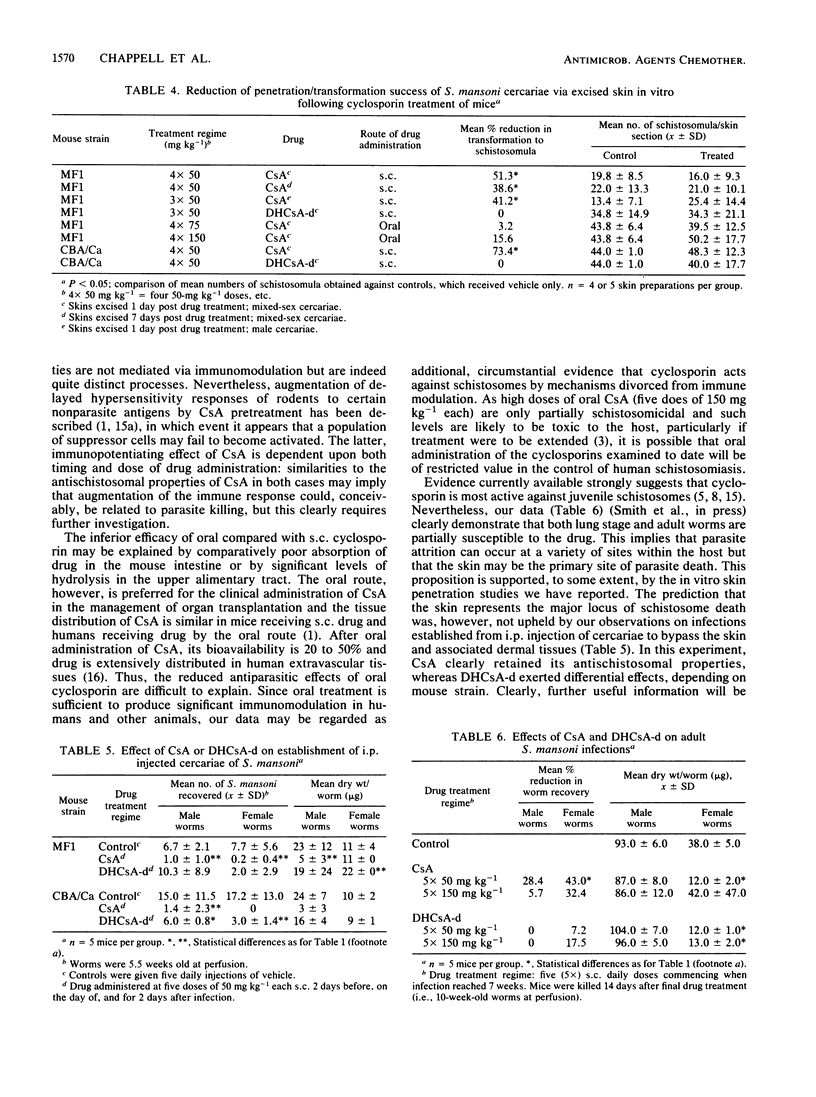
The prophylactic and therapeutic effects of cyclosporin A (CsA) against percutaneous Schistosoma mansoni infection in MF1 mice were dose related and dependent on the temporal relationship between drug administration and infection. Antischistosomal activity, assessed by worm recovery from the host 6 weeks after infection, was most effective (complete worm elimination) when CsA was administered at the time of infection. Oral administration of CsA was less effective than subcutaneous injection, and no prophylactic activity was demonstrated by the former route. Derivatives of dihydrocyclosporin A and cyclosporin C, which have been reported to exert only poor immunosuppressive activity, exhibited efficacy against S. mansoni similar to that of CsA and were also less effective when given orally. Subcutaneous, but not oral CsA reduced cercarial skin penetration and transformation success; the derivative of dihydrocyclosporin A, however, was without effect. Moreover, CsA, but not the derivative of dihydrocyclosporin A, reduced the number of worms established after intraperitoneal injection of cercariae. These data provide further insight into the antischistosomal activity of cyclosporins, which appears to be distinct from their immunomodulatory properties, since parasite killing was retained both in immunologically disparate mice and with poorly immunosuppressive cyclosporin derivatives.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge R. D., Thomson A. W. Factors influencing the enhancement of delayed-type hypersensitivity to ovalbumin by cyclosporin A in the guinea pig: possible role of suppressor cells. Int Arch Allergy Appl Immunol. 1986;81(1):17–23. doi: 10.1159/000234101. [DOI] [PubMed] [Google Scholar]
- Belitsky P., Ghose T., Givner M., Rowden G., Pope B. Tissue distribution of cyclosporine A in the mouse: a clue to toxicity? Clin Nephrol. 1986;25 (Suppl 1):S27–S29. [PubMed] [Google Scholar]
- Belosevic M., Faubert G. M., MacLean J. D. The effects of cyclosporin A on the course of infection with Giardia muris in mice. Am J Trop Med Hyg. 1986 May;35(3):496–500. doi: 10.4269/ajtmh.1986.35.496. [DOI] [PubMed] [Google Scholar]
- Bout D. T., Deslee D., Capron A. R. Protection against schistosomiasis produced by cyclosporin A. Am J Trop Med Hyg. 1984 Jan;33(1):185–186. doi: 10.4269/ajtmh.1984.33.185. [DOI] [PubMed] [Google Scholar]
- Bout D., Deslèe D., Capron A. Antischistosomal effect of cyclosporin A: cure and prevention of mouse and rat schistosomiasis mansoni. Infect Immun. 1986 Jun;52(3):823–827. doi: 10.1128/iai.52.3.823-827.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bout D., Haque A., Capron A. Filaricidal effects of cyclosporin-A against Dipetalonema viteae in Mastomys natalensis. Trans R Soc Trop Med Hyg. 1984;78(5):670–671. doi: 10.1016/0035-9203(84)90236-0. [DOI] [PubMed] [Google Scholar]
- Bueding E., Hawkins J., Cha Y. N. Antischistosomal effects of cyclosporin A. Agents Actions. 1981 Jul;11(4):380–383. doi: 10.1007/BF01982474. [DOI] [PubMed] [Google Scholar]
- Clegg J. A., Smithers S. R. The effects of immune rhesus monkey serum on schistosomula of Schistosoma mansoni during cultivation in vitro. Int J Parasitol. 1972 Mar;2(1):79–98. doi: 10.1016/0020-7519(72)90036-7. [DOI] [PubMed] [Google Scholar]
- Mack D. G., McLeod R. New micromethod to study the effect of antimicrobial agents on Toxoplasma gondii: comparison of sulfadoxine and sulfadiazine individually and in combination with pyrimethamine and study of clindamycin, metronidazole, and cyclosporin A. Antimicrob Agents Chemother. 1984 Jul;26(1):26–30. doi: 10.1128/aac.26.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe R. E., Remington J. S., Araujo F. G. In vivo and in vitro effects of cyclosporin A on Trypanosoma cruzi. Am J Trop Med Hyg. 1985 Sep;34(5):861–865. doi: 10.4269/ajtmh.1985.34.861. [DOI] [PubMed] [Google Scholar]
- Nickell S. P., Scheibel L. W., Cole G. A. Inhibition by cyclosporin A of rodent malaria in vivo and human malaria in vitro. Infect Immun. 1982 Sep;37(3):1093–1100. doi: 10.1128/iai.37.3.1093-1100.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson L. A., Lindblad R., Olling S., Ouchterlony O. The effect of cyclosporin A on the course of murine infection by Schistosoma mansoni. Parasite Immunol. 1985 Jan;7(1):19–27. doi: 10.1111/j.1365-3024.1985.tb00476.x. [DOI] [PubMed] [Google Scholar]
- Thommen-Scott K. Antimalarial activity of cyclosporin A. Agents Actions. 1981 Dec;11(6-7):770–773. doi: 10.1007/BF01978803. [DOI] [PubMed] [Google Scholar]
- Thomson A. W., Smith S. W., Chappell L. H. Cyclosporin A: immune suppressant and antiparasitic agent. Parasitol Today. 1986 Oct;2(10):288–290. doi: 10.1016/0169-4758(86)90141-9. [DOI] [PubMed] [Google Scholar]
- Webster L. M., Thomson A. W. Cyclosporin A prevents suppression of delayed-type hypersensitivity in mice immunized with high-dose sheep erythrocytes. Immunology. 1987 Mar;60(3):409–414. [PMC free article] [PubMed] [Google Scholar]
- Wenger R. Cyclosporine and analogues: structural requirements for immunosuppressive activity. Transplant Proc. 1986 Dec;18(6 Suppl 5):213–218. [PubMed] [Google Scholar]
- Wood A. J., Lemaire M. Pharmacologic aspects of cyclosporine therapy: pharmacokinetics. Transplant Proc. 1985 Aug;17(4 Suppl 1):27–32. [PubMed] [Google Scholar]


