Abstract
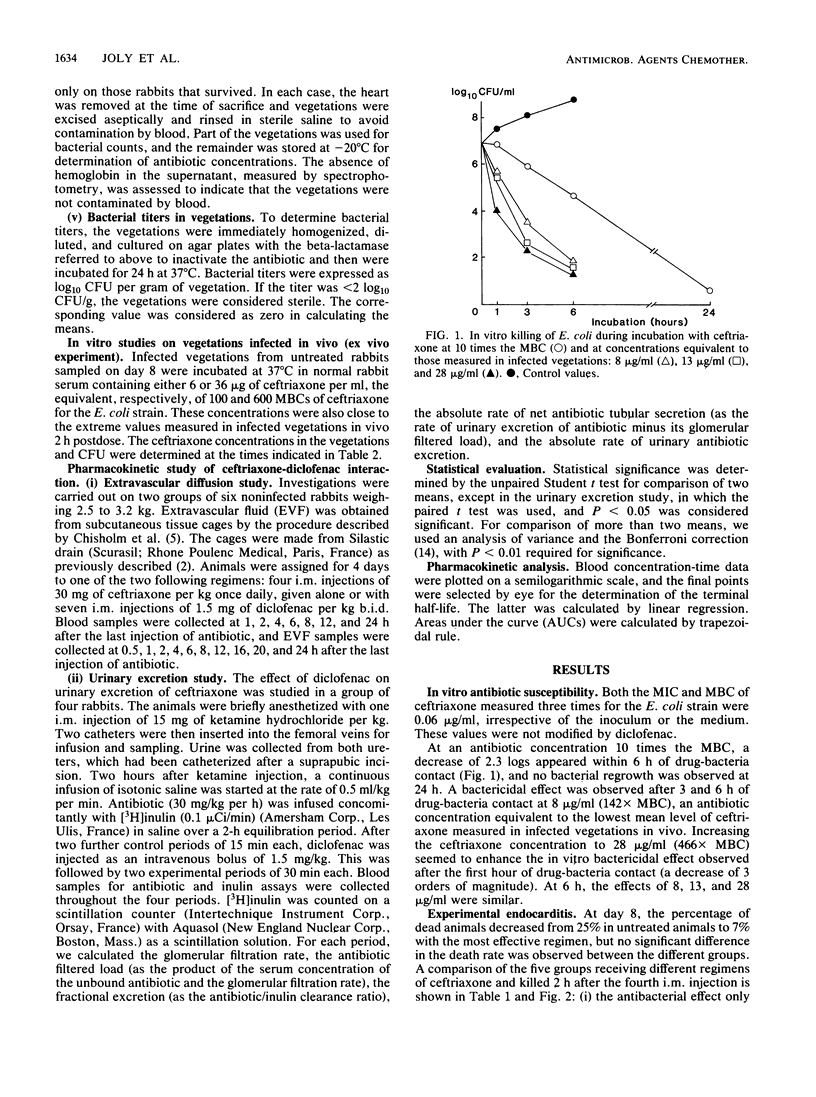
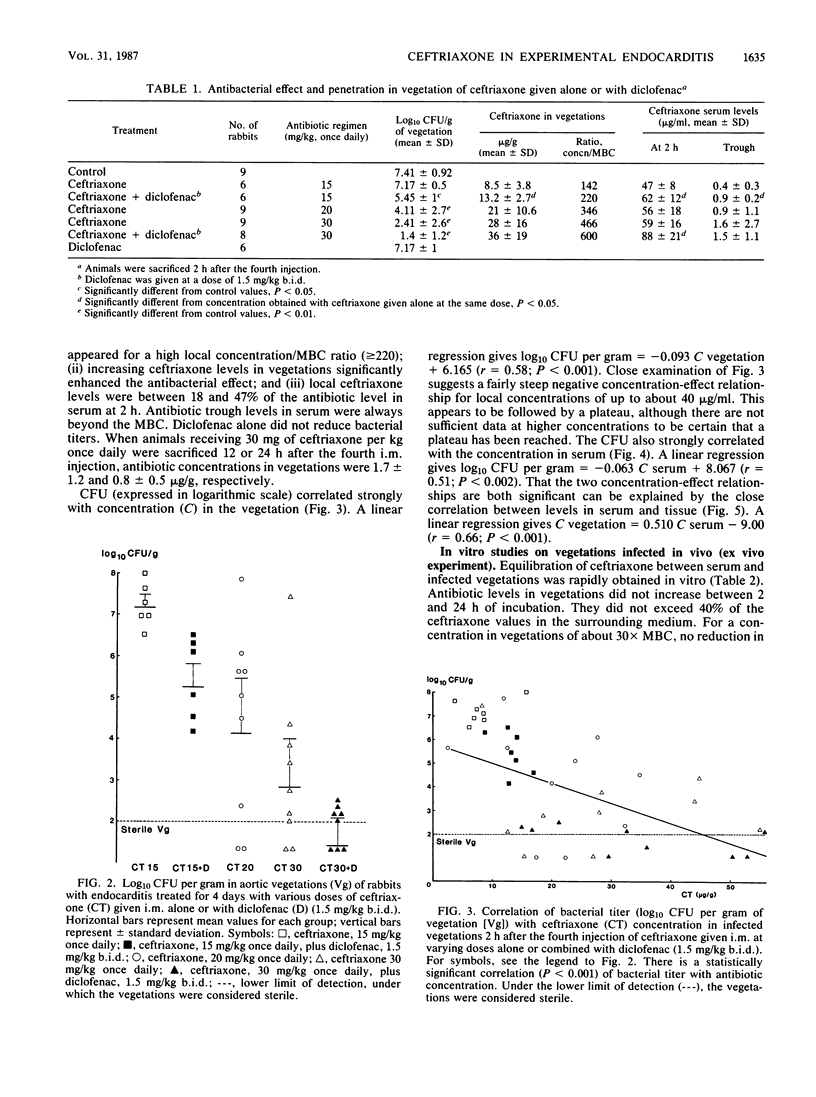
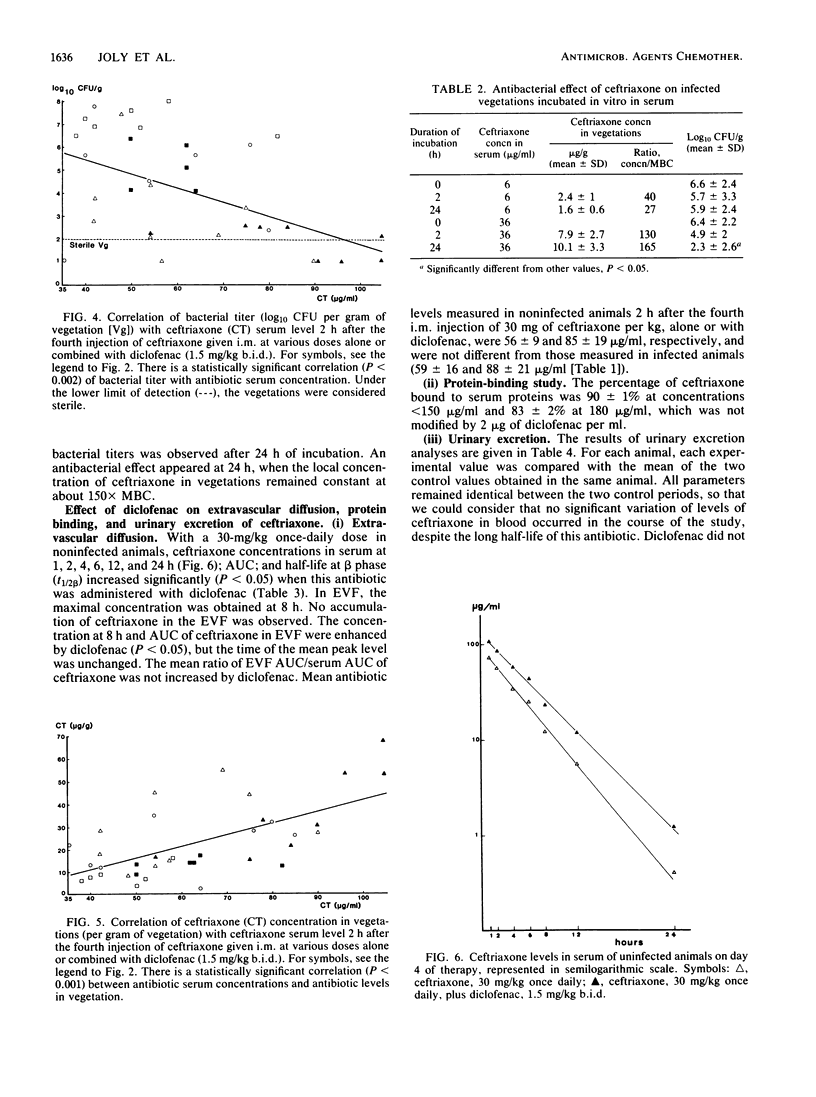
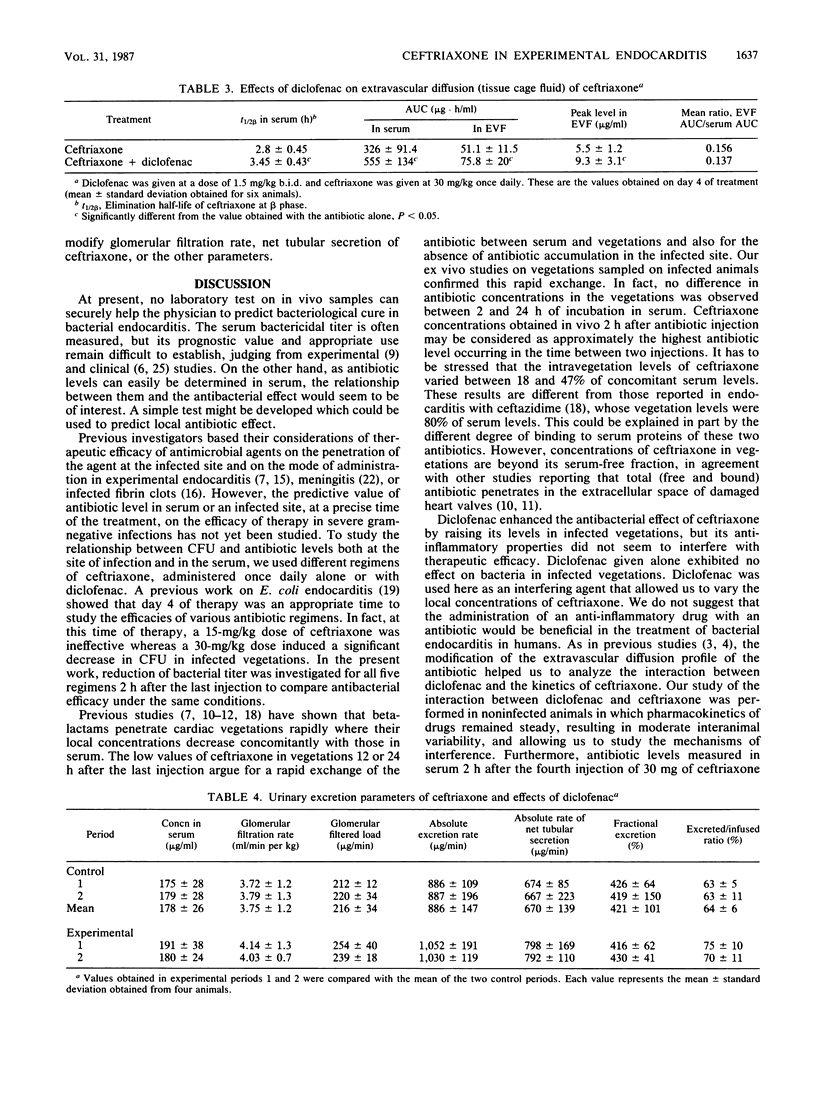
In a rabbit model of Escherichia coli endocarditis, we studied the penetration into infected vegetations and the antibacterial effect of ceftriaxone. Ceftriaxone was given at different dosages, alone or with an interfering agent, diclofenac, a nonsteroidal anti-inflammatory drug, to determine the predictive value of the antibiotic levels in serum or infected vegetations on the antibacterial efficacy. Diclofenac increased the serum terminal half-life of ceftriaxone and increased its extravascular diffusion in tissue cage fluid, as well as in infected vegetations, allowing us to obtain various antibiotic concentrations in the infected site. Two hours after the fourth injection, around the time of peak level in serum, we observed a linear relationship between (i) serum and local antibiotic levels in vegetations, (ii) local antibiotic levels in a range of 142 to 600 X MBC and bacterial titer (log10 CFU/g) in vegetations, and (iii) serum antibiotic levels in a range of 800 to 1,400X MBC and bacterial titer in vegetations. In vivo, antibacterial effect was obtained only with high antibiotic levels in vegetations (greater than or equal to 220X MBC). This was confirmed by incubating vegetations sampled from infected animals in rabbit serum containing ceftriaxone (ex vivo experiment). Given once daily at a therapeutic dosage (30 mg/kg) for 4 days, ceftriaxone exhibited good efficacy (log10 CFU/g of vegetation = 2.41 +/- 2.7 versus 7.41 +/- 0.92 in control animals) and prevented regrowth of bacteria until 24 h after the last injection. We concluded that (i) provided the dose is sufficient, a long-acting cephalosporin can prove effective in severe gram-negative infections even when given infrequently, and (ii) serum antibiotic levels around the peak value, reflecting high effective local levels, could predict the therapeutic efficacy and represent a simple test to monitor the clinical course of a severe infectious process.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouvet A., Cremieux A. C., Contrepois A., Vallois J. M., Lamesch C., Carbon C. Comparison of penicillin and vancomycin, individually and in combination with gentamicin and amikacin, in the treatment of experimental endocarditis induced by nutritionally variant streptococci. Antimicrob Agents Chemother. 1985 Nov;28(5):607–611. doi: 10.1128/aac.28.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon C., Contrepois A., Brion N., Lamotte-Barrillon S. Penetration of cefazolin, cephaloridine, and cefamandole into interstitial fluid in rabbits. Antimicrob Agents Chemother. 1977 Apr;11(4):594–598. doi: 10.1128/aac.11.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon C., Contrepois A., Nivoche Y., Grandjean M., Decourt S., Chau N. P. Effects of phenylbutazone on extravascular diffusion, protein binding and urinary excretion of cefazolin in rabbits. J Pharmacol Exp Ther. 1981 Aug;218(2):537–543. [PubMed] [Google Scholar]
- Carbon C., Dromer F., Brion N., Cremieux A. C., Contrepois A. Renal disposition of ceftazidime illustrated by interferences by probenecid, furosemide, and indomethacin in rabbits. Antimicrob Agents Chemother. 1984 Sep;26(3):373–377. doi: 10.1128/aac.26.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm G. D., Waterworth P. M., Calnan J. S., Garrod L. P. Concentration of antibacterial agents in interstitial tissue fluid. Br Med J. 1973 Mar 10;1(5853):569–573. doi: 10.1136/bmj.1.5853.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman D. L., Horwitz R. I., Andriole V. T. Association between serum inhibitory and bactericidal concentrations and therapeutic outcome in bacterial endocarditis. Am J Med. 1982 Aug;73(2):260–267. doi: 10.1016/0002-9343(82)90188-7. [DOI] [PubMed] [Google Scholar]
- Contrepois A., Vallois J. M., Garaud J. J., Pangon B., Mohler J., Meulemans A., Carbon C. Kinetics and bactericidal effect of gentamicin and latamoxef (moxalactam) in experimental Escherichia coli endocarditis. J Antimicrob Chemother. 1986 Feb;17(2):227–237. doi: 10.1093/jac/17.2.227. [DOI] [PubMed] [Google Scholar]
- Decazes J. M., Ernst J. D., Sande M. A. Correlation of in vitro time-kill curves and kinetics of bacterial killing in cerebrospinal fluid during ceftriaxone therapy of experimental Escherichia coli meningitis. Antimicrob Agents Chemother. 1983 Oct;24(4):463–467. doi: 10.1128/aac.24.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durack D. T., Pelletier L. L., Petersdorf R. G. Chemotherapy of experimental streptococcal endocarditis. II. Synergism between penicillin and streptomycin against penicillin-sensitive streptococci. J Clin Invest. 1974 Mar;53(3):829–833. doi: 10.1172/JCI107622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick B. C., Gengo F. M., Schentag J. J. Moxalactam penetration into normal heart valve, cardiac vegetations, and myocardium in relation to protein binding and physiological distribution spaces. Antimicrob Agents Chemother. 1984 Aug;26(2):228–230. doi: 10.1128/aac.26.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengo F. M., Schentag J. J. Methicillin distribution in serum and extravascular fluid and its relevance to normal and damaged heart valves. Antimicrob Agents Chemother. 1981 May;19(5):836–841. doi: 10.1128/aac.19.5.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengo F. M., Schentag J. J. Rate of methicillin penetration into normal heart valve and experimental endocarditis lesions. Antimicrob Agents Chemother. 1982 Mar;21(3):456–459. doi: 10.1128/aac.21.3.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber A. U., Brugger H. P., Feller C., Stritzko T., Stalder B. Antibiotic therapy of infections due to Pseudomonas aeruginosa in normal and granulocytopenic mice: comparison of murine and human pharmacokinetics. J Infect Dis. 1986 Jan;153(1):90–97. doi: 10.1093/infdis/153.1.90. [DOI] [PubMed] [Google Scholar]
- Ingerman M. J., Pitsakis P. G., Rosenberg A. F., Levison M. E. The importance of pharmacodynamics in determining the dosing interval in therapy for experimental pseudomonas endocarditis in the rat. J Infect Dis. 1986 Apr;153(4):707–714. doi: 10.1093/infdis/153.4.707. [DOI] [PubMed] [Google Scholar]
- Lavoie G. Y., Bergeron M. G. Influence of four modes of administration on penetration of aztreonam, cefuroxime, and ampicillin into interstitial fluid and fibrin clots and on in vivo efficacy against Haemophilus influenzae. Antimicrob Agents Chemother. 1985 Sep;28(3):404–412. doi: 10.1128/aac.28.3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. S., Komarmy L. New method for detecting in vitro inactivation of penicillins by Haemophilus influenzae and Staphlycoccus aureus. Antimicrob Agents Chemother. 1976 Sep;10(3):564–566. doi: 10.1128/aac.10.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColm A. A., Ryan D. M. Comparative pharmacokinetics of ceftazidime in fibrin clots and cardiac vegetations in rabbits with Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1985 Jun;27(6):925–927. doi: 10.1128/aac.27.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangon B., Joly V., Vallois J. M., Abel L., Buré A., Brion N., Contrepois A., Carbon C. Comparative efficacy of cefotiam, cefmenoxime, and ceftriaxone in experimental endocarditis and correlation with pharmacokinetics and in vitro efficacy. Antimicrob Agents Chemother. 1987 Apr;31(4):518–522. doi: 10.1128/aac.31.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel I. H., Chen S., Parsonnet M., Hackman M. R., Brooks M. A., Konikoff J., Kaplan S. A. Pharmacokinetics of ceftriaxone in humans. Antimicrob Agents Chemother. 1981 Nov;20(5):634–641. doi: 10.1128/aac.20.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock A. A., Tee P. E., Patel I. H., Spicehandler J., Simberkoff M. S., Rahal J. J., Jr Pharmacokinetic characteristics of intravenous ceftriaxone in normal adults. Antimicrob Agents Chemother. 1982 Nov;22(5):816–823. doi: 10.1128/aac.22.5.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaad U. B., McCracken G. H., Jr, Loock C. A., Thomas M. L. Pharmacokinetics and bacteriologic efficacy of moxalactam, cefotaxime, cefoperazone, and rocephin in experimental bacterial meningitis. J Infect Dis. 1981 Feb;143(2):156–163. doi: 10.1093/infdis/143.2.156. [DOI] [PubMed] [Google Scholar]
- Scheld W. M., Sande M. A. Bactericidal versus bacteriostatic antibiotic therapy of experimental pneumococcal meningitis in rabbits. J Clin Invest. 1983 Mar;71(3):411–419. doi: 10.1172/JCI110785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton C. W., Weinstein M. P., Reller L. B. Correlation of serum bactericidal activity with antimicrobial agent level and minimal bactericidal concentration. J Infect Dis. 1982 Feb;145(2):160–168. doi: 10.1093/infdis/145.2.160. [DOI] [PubMed] [Google Scholar]
- Weinstein M. P., Stratton C. W., Ackley A., Hawley H. B., Robinson P. A., Fisher B. D., Alcid D. V., Stephens D. S., Reller L. B. Multicenter collaborative evaluation of a standardized serum bactericidal test as a prognostic indicator in infective endocarditis. Am J Med. 1985 Feb;78(2):262–269. doi: 10.1016/0002-9343(85)90436-x. [DOI] [PubMed] [Google Scholar]


