Abstract
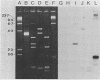
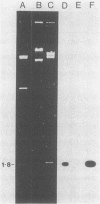
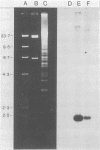
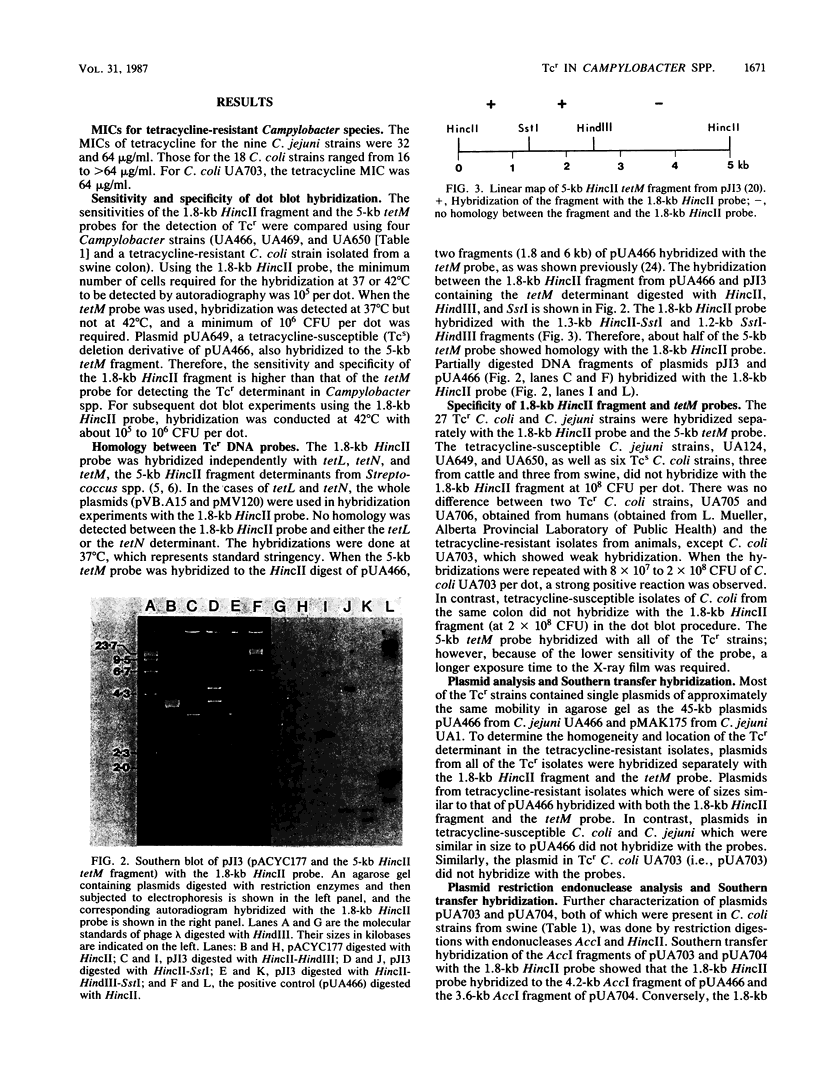
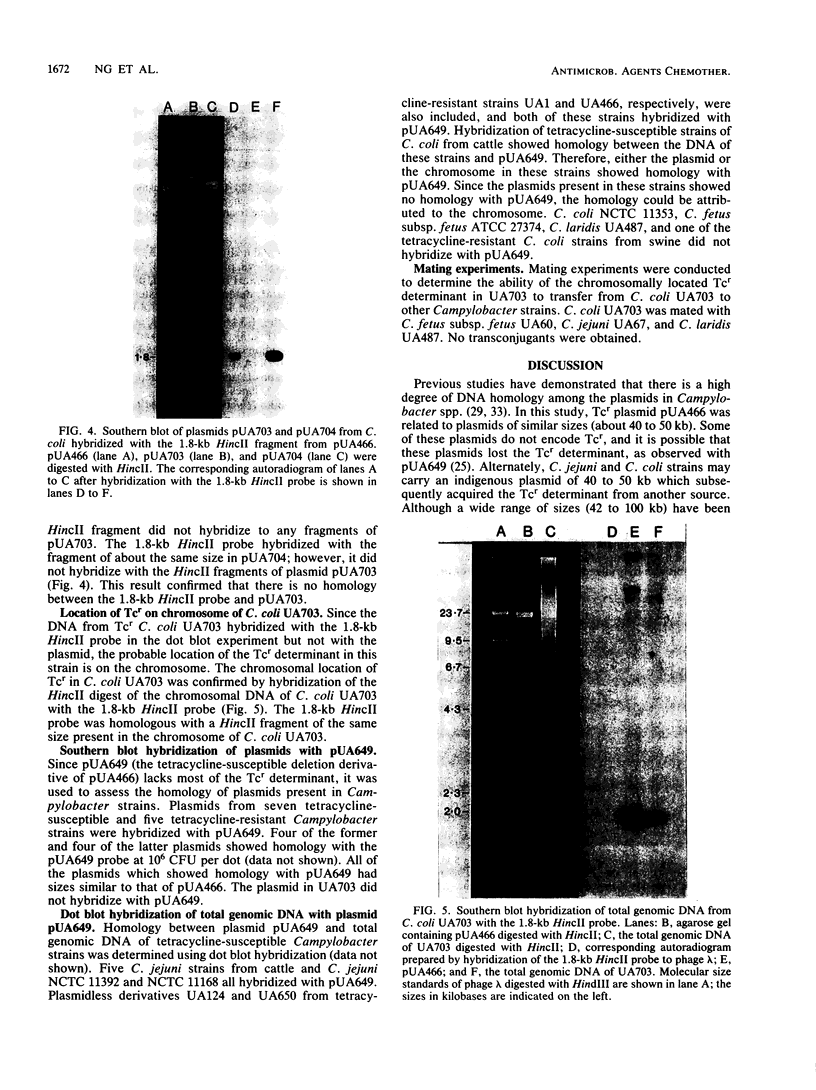
Tetracycline-resistant strains of Campylobacter jejuni and Campylobacter coli from swine and cattle colons were isolated and characterized by hybridization with DNA probes. A probe consisting of the 1.8-kilobase (kb) HincII fragment from pUA466 was highly specific for the detection of tetracycline resistance (Tcr) in C. jejuni and C. coli. The 5-kb tetM DNA probe from Streptococcus agalactiae plasmid pJI3 which has homology with the 1.8-kb HincII fragment from pUA466 could also be used to detect Tcr Campylobacter strains. However, the tetM probe had a much lower sensitivity and required a lower stringency of hybridization. Therefore, the 1.8-kb HincII fragment appeared to be more appropriate for the classification of Tcr in Campylobacter spp. No homology was detected between the Tcr determinant from Campylobacter spp. and the tetL and tetN probes from Streptococcus spp. DNA homology was demonstrated between pUA649, a derivative of plasmid pUA466 which had lost most of the Tcr region, and Tcr plasmids from C. jejuni and C. coli isolated from animal and human sources. There was also homology between pUA649 and the chromosomes of C. jejuni and C. coli strains. In this study, all but one of the tetracycline-resistant C. coli and C. jejuni strains contained plasmids of approximately 50 kb which hybridized with the 1.8-kb HincII probe. In one C. coli strain (UA703), Tcr appeared to be chromosomally mediated.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury W. C., Marko M. A., Hennessy J. N., Penner J. L. Occurrence of plasmid DNA in serologically defined strains of Campylobacter jejuni and Campylobacter coli. Infect Immun. 1983 May;40(2):460–463. doi: 10.1128/iai.40.2.460-463.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury W. C., Munroe D. L. Occurrence of plasmids and antibiotic resistance among Campylobacter jejuni and Campylobacter coli isolated from healthy and diarrheic animals. J Clin Microbiol. 1985 Sep;22(3):339–346. doi: 10.1128/jcm.22.3.339-346.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett V. Identification of tetracycline-resistant R-plasmids in Streptococcus agalactiae (group B). Antimicrob Agents Chemother. 1980 Nov;18(5):753–760. doi: 10.1128/aac.18.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett V., Inamine J., Rajagopalan S. Heterogeneity of tetracycline resistance determinants in Streptococcus. J Bacteriol. 1982 Mar;149(3):995–1004. doi: 10.1128/jb.149.3.995-1004.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Lambert T., Gerbaud G., Trieu-Cuot P., Courvalin P. Structural relationship between the genes encoding 3'-aminoglycoside phosphotransferases in Campylobacter and in gram-positive cocci. Ann Inst Pasteur Microbiol. 1985 Sep-Oct;136B(2):135–150. doi: 10.1016/s0769-2609(85)80040-5. [DOI] [PubMed] [Google Scholar]
- Levy S. B., FitzGerald G. B., Macone A. B. Changes in intestinal flora of farm personnel after introduction of a tetracycline-supplemented feed on a farm. N Engl J Med. 1976 Sep 9;295(11):583–588. doi: 10.1056/NEJM197609092951103. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Thermal renaturation of deoxyribonucleic acids. J Mol Biol. 1961 Oct;3:585–594. doi: 10.1016/s0022-2836(61)80023-5. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Trieu-Cuot P., Courvalin P. Nucleotide sequence of the tetM tetracycline resistance determinant of the streptococcal conjugative shuttle transposon Tn1545. Nucleic Acids Res. 1986 Sep 11;14(17):7047–7058. doi: 10.1093/nar/14.17.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConaughy B. L., Laird C. D., McCarthy B. J. Nucleic acid reassociation in formamide. Biochemistry. 1969 Aug;8(8):3289–3295. doi: 10.1021/bi00836a024. [DOI] [PubMed] [Google Scholar]
- Mendez B., Tachibana C., Levy S. B. Heterogeneity of tetracycline resistance determinants. Plasmid. 1980 Mar;3(2):99–108. doi: 10.1016/0147-619x(80)90101-8. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Johnson S. R., Biddle J. W., Roberts M. C. High-level tetracycline resistance in Neisseria gonorrhoeae is result of acquisition of streptococcal tetM determinant. Antimicrob Agents Chemother. 1986 Nov;30(5):664–670. doi: 10.1128/aac.30.5.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley S. L., Echeverria P., Seriwatana J., Tirapat C., Chaicumpa W., Sakuldaipeara T., Falkow S. Identification of enterotoxigenic Escherichia coli by colony hybridization using three enterotoxin gene probes. J Infect Dis. 1982 Jun;145(6):863–869. doi: 10.1093/infdis/145.6.863. [DOI] [PubMed] [Google Scholar]
- Ng L. K., Stiles M. E., Taylor D. E. Inhibition of Campylobacter coli and Campylobacter jejuni by antibiotics used in selective growth media. J Clin Microbiol. 1985 Oct;22(4):510–514. doi: 10.1128/jcm.22.4.510-514.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Moseley S. L., Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981 Feb;31(2):775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. C., Hillier S. L., Hale J., Holmes K. K., Kenny G. E. Tetracycline resistance and tetM in pathogenic urogenital bacteria. Antimicrob Agents Chemother. 1986 Nov;30(5):810–812. doi: 10.1128/aac.30.5.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. C., Kenny G. E. Dissemination of the tetM tetracycline resistance determinant to Ureaplasma urealyticum. Antimicrob Agents Chemother. 1986 Feb;29(2):350–352. doi: 10.1128/aac.29.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildkraut C. Dependence of the melting temperature of DNA on salt concentration. Biopolymers. 1965;3(2):195–208. doi: 10.1002/bip.360030207. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taylor D. E., Brose E. C. Restriction endonuclease mapping of R27 (TP117), an incompatibility group HI subgroup 1 plasmid from Salmonella typhimurium. Plasmid. 1985 Jan;13(1):75–77. doi: 10.1016/0147-619x(85)90058-7. [DOI] [PubMed] [Google Scholar]
- Taylor D. E., Chang N., Garner R. S., Sherburne R., Mueller L. Incidence of antibiotic resistance and characterization of plasmids in Campylobacter jejuni strains isolated from clinical sources in Alberta, Canada. Can J Microbiol. 1986 Jan;32(1):28–32. doi: 10.1139/m86-006. [DOI] [PubMed] [Google Scholar]
- Taylor D. E., De Grandis S. A., Karmali M. A., Fleming P. C. Transmissible plasmids from Campylobacter jejuni. Antimicrob Agents Chemother. 1981 May;19(5):831–835. doi: 10.1128/aac.19.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., Garner R. S., Allan B. J. Characterization of tetracycline resistance plasmids from Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother. 1983 Dec;24(6):930–935. doi: 10.1128/aac.24.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., Hiratsuka K., Ray H., Manavathu E. K. Characterization and expression of a cloned tetracycline resistance determinant from Campylobacter jejuni plasmid pUA466. J Bacteriol. 1987 Jul;169(7):2984–2989. doi: 10.1128/jb.169.7.2984-2989.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., Newell D. G., Pearson A. D. Incidence of plasmid DNA in strains of Campylobacter jejuni isolated from stool specimens at 37 C and 43 C. J Infect Dis. 1983 May;147(5):965–966. doi: 10.1093/infdis/147.5.965. [DOI] [PubMed] [Google Scholar]
- Taylor D. E. Plasmid-mediated tetracycline resistance in Campylobacter jejuni: expression in Escherichia coli and identification of homology with streptococcal class M determinant. J Bacteriol. 1986 Mar;165(3):1037–1039. doi: 10.1128/jb.165.3.1037-1039.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F. C., Bronsdon M. A., Gordon K. P., Plorde J. J. Isolation of plasmids encoding tetracycline resistance from Campylobacter jejuni strains isolated from simians. Antimicrob Agents Chemother. 1983 Feb;23(2):320–322. doi: 10.1128/aac.23.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F. C., Williams S., Gordon K. P., Nolan C., Plorde J. J. Survey of plasmids and resistance factors in Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother. 1985 Jan;27(1):37–41. doi: 10.1128/aac.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totten P. A., Holmes K. K., Handsfield H. H., Knapp J. S., Perine P. L., Falkow S. DNA hybridization technique for the detection of Neisseria gonorrhoeae in men with urethritis. J Infect Dis. 1983 Sep;148(3):462–471. doi: 10.1093/infdis/148.3.462. [DOI] [PubMed] [Google Scholar]