Abstract
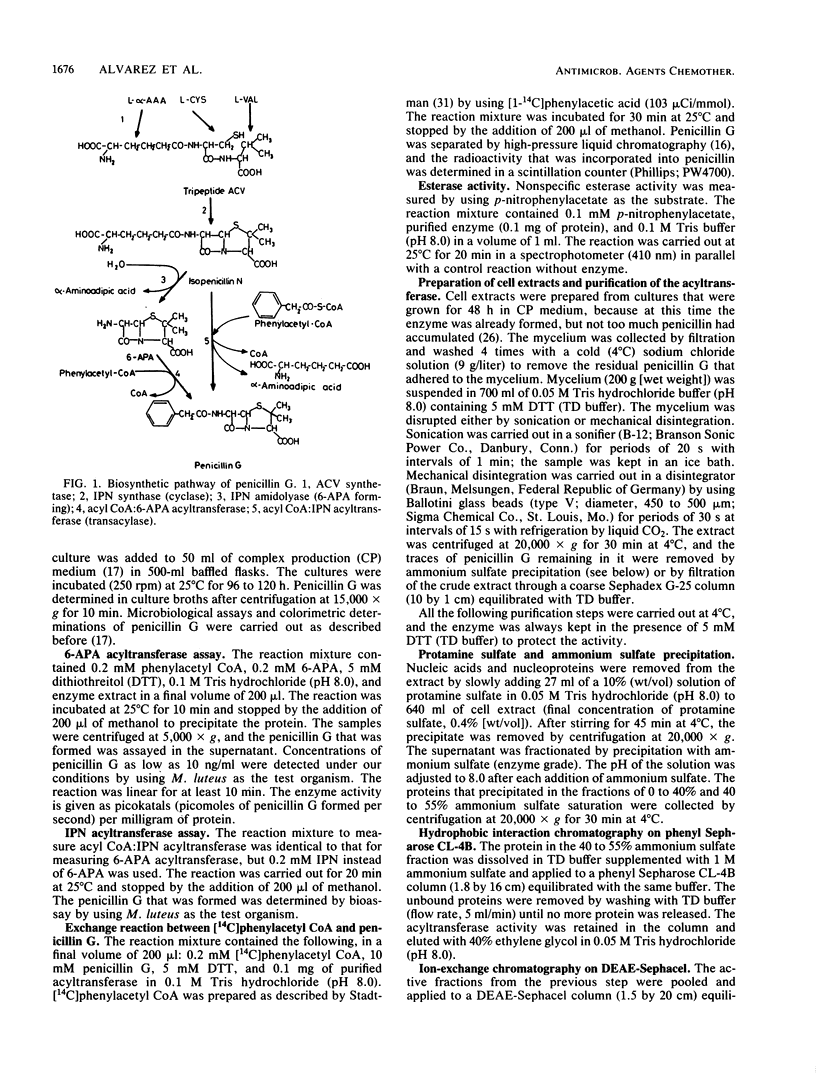
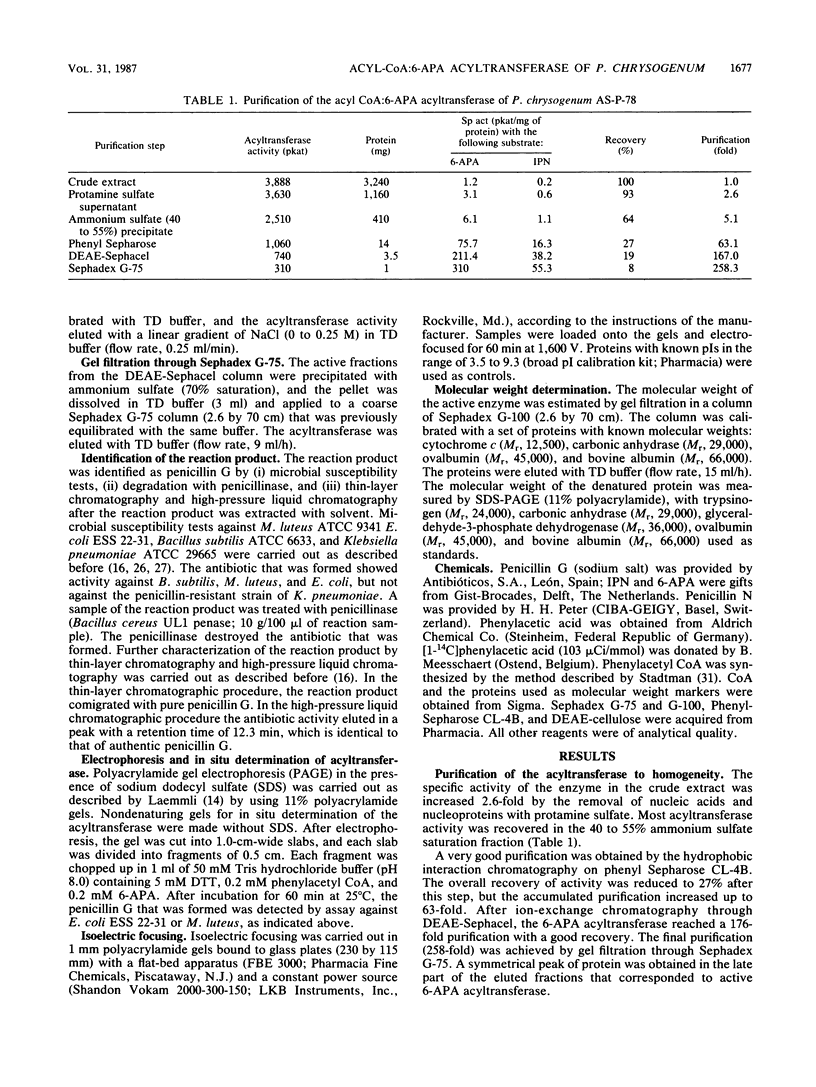
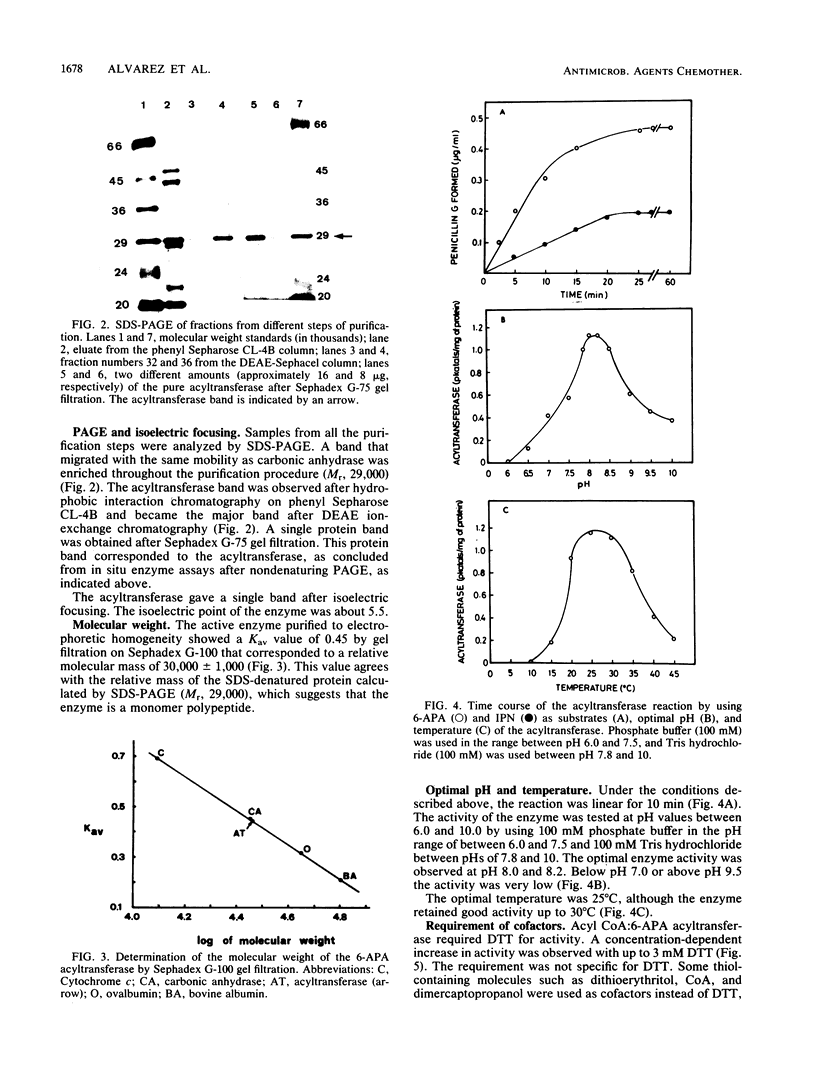
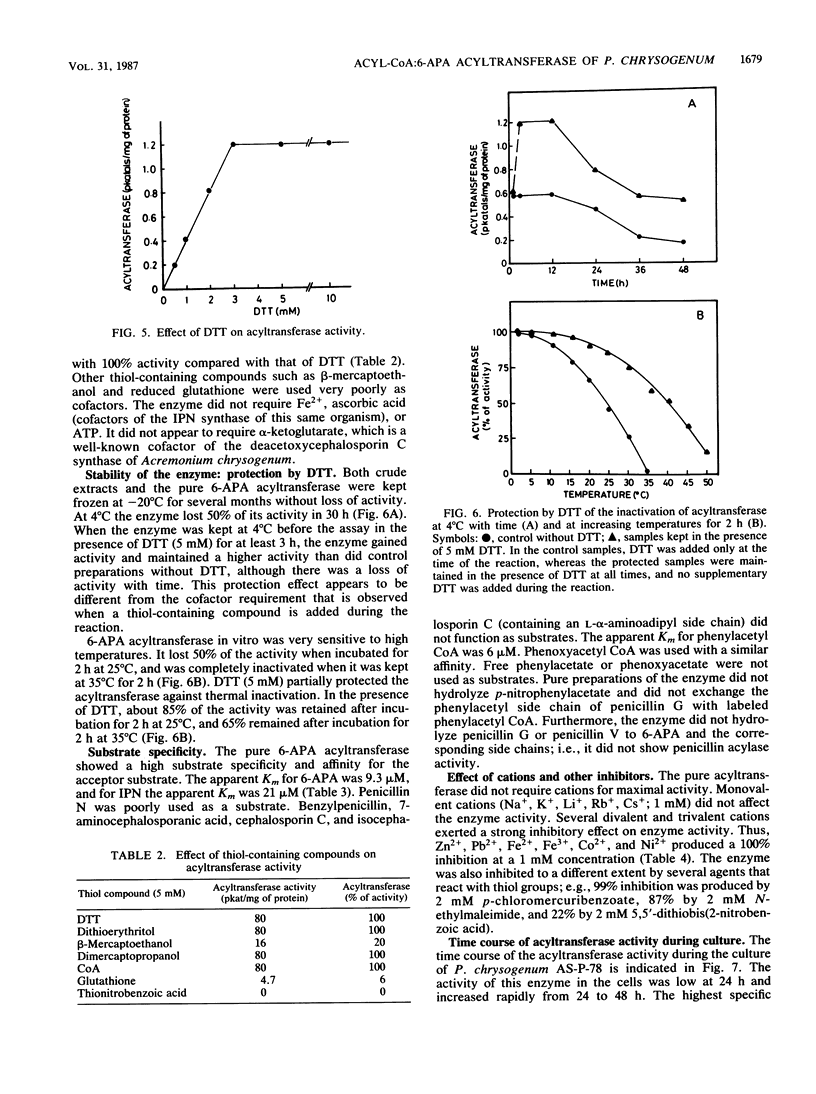
The acyl coenzyme A (CoA):6-aminopenicillanic acid (6-APA) acyltransferase of Penicillium chrysogenum AS-P-78 was purified to homogeneity, as concluded by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and isoelectric focusing. The enzyme is a monomer with a molecular weight of 30,000 +/- 1,000 and a pI of about 5.5. The optimal pH and temperature were 8.0 and 25 degrees C, respectively. This enzyme converts 6-APA into penicillin by using phenylacetyl CoA or phenoxyacetyl CoA as acyl donors. The pure enzyme showed a high specificity and affinity for 6-APA and did not accept benzylpenicillin, 7-aminocephalosporanic acid, cephalosporin C, or isocephalosporin C as substrates. The enzyme converted isopenicillin N into penicillin G, although with a lower efficiency than when 6-APA was used as the substrate. It did not show penicillin G acylase activity. The acyl CoA:6-APA acyltransferase required dithiothreitol or other thiol-containing compounds, and it was protected by thiol-containing reagents against thermal inactivation. The acyltransferase was inhibited by several divalent and trivalent cations and by p-chloromercuribenzoate and N-ethylmaleimide. The activity was absent in four different mutants that were blocked in penicillin biosynthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BATCHELOR F. R., DOYLE F. P., NAYLER J. H., ROLINSON G. N. Synthesis of penicillin: 6-aminopenicillanic acid in penicillin fermentations. Nature. 1959 Jan 24;183(4656):257–258. doi: 10.1038/183257b0. [DOI] [PubMed] [Google Scholar]
- Baldwin J. E., Gagnon J., Ting H. N-terminal amino acid sequence and some properties of isopenicillin-N synthetase from Cephalosporium acremonium. FEBS Lett. 1985 Sep 2;188(2):253–256. doi: 10.1016/0014-5793(85)80382-3. [DOI] [PubMed] [Google Scholar]
- Brundidge S. P., Gaeta F. C., Hook D. J., Sapino C., Jr, Elander R. P., Morin R. B. Association of 6-oxo-piperidine-2-carboxylic acid with penicillin V. Production on Penicillium chrysogenum fermentations. J Antibiot (Tokyo) 1980 Nov;33(11):1348–1351. doi: 10.7164/antibiotics.33.1348. [DOI] [PubMed] [Google Scholar]
- Brunner R., Rohr M. Phenacyl:coenzyme A ligase. Methods Enzymol. 1975;43:476–481. doi: 10.1016/0076-6879(75)43107-x. [DOI] [PubMed] [Google Scholar]
- Brunner R., Röhr M., Zinner M. Zur Biosynthese des Penicillins. Untersuchungen zur enzymatischen Aktivierung von Phenylessigsäure und Phenoxyessigsäure sowie zur Bildung von Penicillin aus 6-Amino-penicillansäure und aktivierter Seitenkettensäure durch Mycelhomogenate und zellfreie Extrakte von Penicillium chrysogenum. Hoppe Seylers Z Physiol Chem. 1968 Jan;349(1):95–103. doi: 10.1515/bchm2.1968.349.1.95. [DOI] [PubMed] [Google Scholar]
- Cole M. Formation of 6-aminopenicillanic acid, penicillins, and penicillin acylase by various fungi. Appl Microbiol. 1966 Jan;14(1):98–104. doi: 10.1128/am.14.1.98-104.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson R. C., Bennett R. E. Penicillin acylase activity of Penicillium chrysogenum. Appl Microbiol. 1965 Sep;13(5):738–742. doi: 10.1128/am.13.5.738-742.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett P. A., Usher J. J., Abraham E. P. Behaviour of tritium-labelled isopenicillin N and 6-aminopenicillanic acid as potential penicillin precursors in an extract of Penicillum chrysogenum. Biochem J. 1975 Dec;151(3):741–746. doi: 10.1042/bj1510741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenbeck S., Brunsberg U. Biosynthesis of penicillins. I. Isolation of a 6-aminopenicillanic acid acyltransferase from Penicillium chrysogenum. Acta Chem Scand. 1968;22(3):1059–1061. doi: 10.3891/acta.chem.scand.22-1059. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luengo J. M., Revilla G., Villanueva J. R., Martín J. F. Lysine regulation of penicillin biosynthesis in low-producing and industrial strains of Penicillium chrysogenum. J Gen Microbiol. 1979 Nov;115(1):207–211. doi: 10.1099/00221287-115-1-207. [DOI] [PubMed] [Google Scholar]
- Pruess D. L., Johnson M. J. Penicillin acyltransferase in Penicillium chrysogenum. J Bacteriol. 1967 Nov;94(5):1502–1508. doi: 10.1128/jb.94.5.1502-1508.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos F. R., López-Nieto M. J., Martín J. F. Isopenicillin N synthetase of Penicillium chrysogenum, an enzyme that converts delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine to isopenicillin N. Antimicrob Agents Chemother. 1985 Mar;27(3):380–387. doi: 10.1128/aac.27.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revilla G., Ramos F. R., López-Nieto M. J., Alvarez E., Martín J. F. Glucose represses formation of delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine and isopenicillin N synthase but not penicillin acyltransferase in Penicillium chrysogenum. J Bacteriol. 1986 Nov;168(2):947–952. doi: 10.1128/jb.168.2.947-952.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson S. M., Belagaje R., Blankenship D. T., Chapman J. L., Perry D., Skatrud P. L., VanFrank R. M., Abraham E. P., Baldwin J. E., Queener S. W. Isolation, sequence determination and expression in Escherichia coli of the isopenicillin N synthetase gene from Cephalosporium acremonium. Nature. 1985 Nov 14;318(6042):191–194. doi: 10.1038/318191a0. [DOI] [PubMed] [Google Scholar]
- Spencer B. The biosynthesis of penicillins: Acylation of 6-aminopenicillanic acid. Biochem Biophys Res Commun. 1968 Apr 19;31(2):170–175. doi: 10.1016/0006-291x(68)90725-0. [DOI] [PubMed] [Google Scholar]
- Vanderhaeghe H., Claesen M., Vlietinck A., Parmentier G. Specificity of penicillin acylase of Fusarium and of Penicillium chrysogenum. Appl Microbiol. 1968 Oct;16(10):1557–1563. doi: 10.1128/am.16.10.1557-1563.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]




