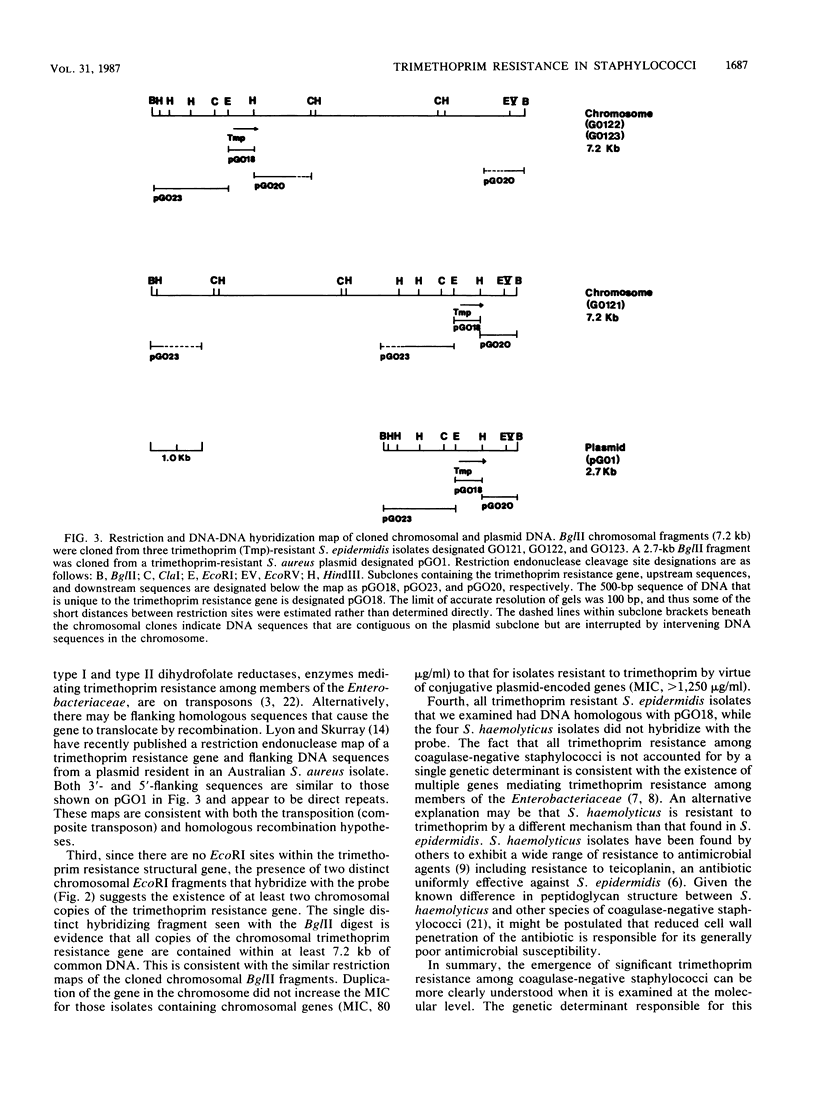
Abstract
A 42% (70 of 167 isolates) incidence of resistance to 20 micrograms of trimethoprim per ml was found among clinical isolates of coagulase-negative staphylococci from two hospitals. A specific trimethoprim resistance gene probe from a conjugative Staphylococcus aereus plasmid was used to investigate the location of the trimethoprim resistance gene among 29 isolates. In 14 trimethoprim-resistant isolates, the probe hybridized with only chromosomal DNA, in 9 it hybridized with only plasmid DNA, and in 1 isolate both plasmid and chromosomal sequences showed hybridization. In five isolates there was no hybridization of the probe with either chromosomal or plasmid DNA. Four of these five nonhybridizing isolates were Staphylococcus haemolyticus. In contrast, all 22 Staphylococcus epidermidis isolates tested hybridized with the probe. The presence of the trimethoprim resistance gene in a chromosomal location was correlated with a lower MIC (median, 80 micrograms/ml) than when it was plasmid encoded (median, 1,250 micrograms/ml). Restriction endonuclease mapping as well as DNA hybridization of cloned plasmid and chromosomal DNA showed that there were 2.7 kilobases of common DNA in the two loci. This included the 500 base pairs of DNA mediating trimethoprim resistance and a total of 2.2 kilobases of 3'- and 5'-flanking sequences. The presence of the same gene and flanking sequences in chromosomal and plasmid locations suggests that the trimethoprim resistance determinant is translocated among different genetic loci.
Full text
PDF

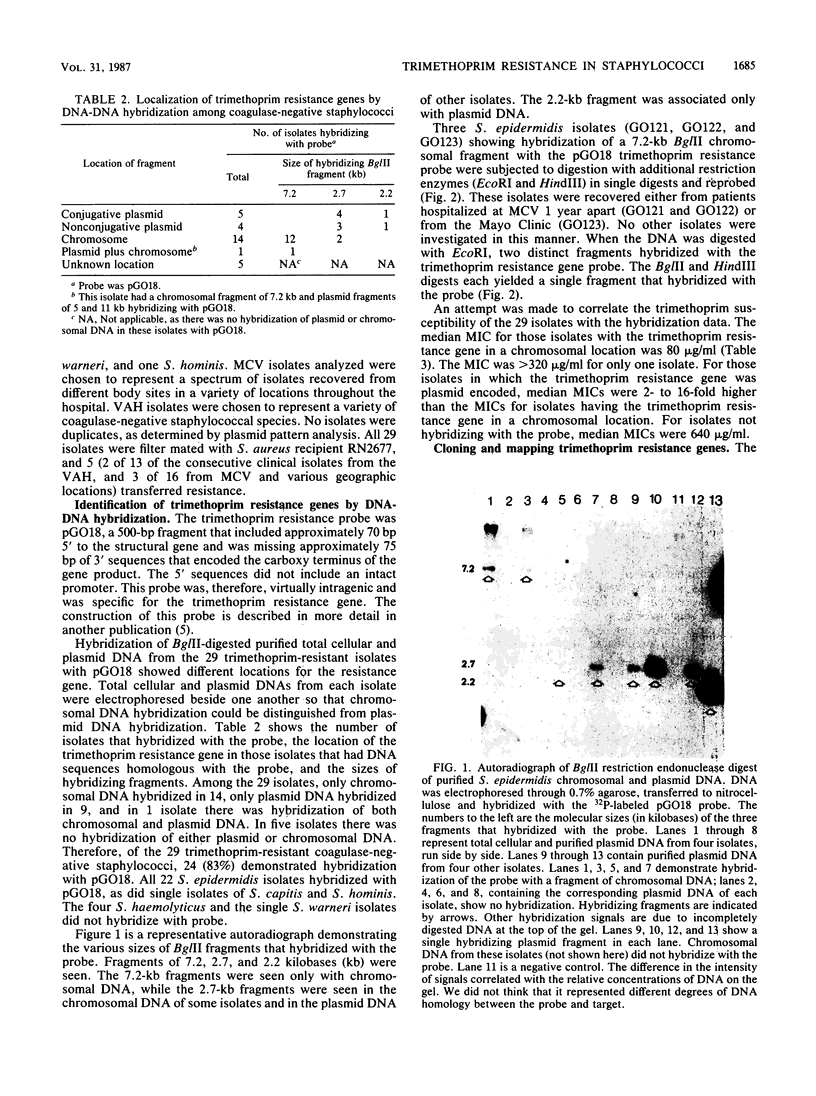
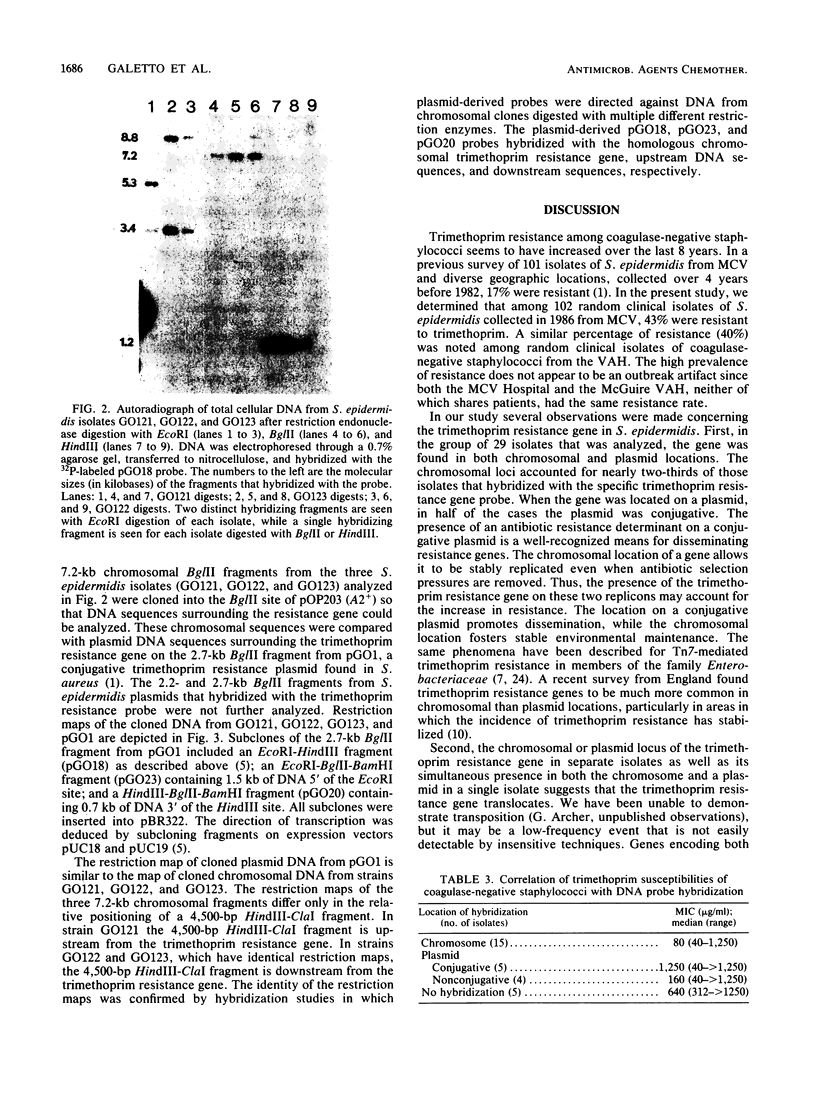

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer G. L., Coughter J. P., Johnston J. L. Plasmid-encoded trimethoprim resistance in staphylococci. Antimicrob Agents Chemother. 1986 May;29(5):733–740. doi: 10.1128/aac.29.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer G. L., Dietrick D. R., Johnston J. L. Molecular epidemiology of transmissible gentamicin resistance among coagulase-negative staphylococci in a cardiac surgery unit. J Infect Dis. 1985 Feb;151(2):243–251. doi: 10.1093/infdis/151.2.243. [DOI] [PubMed] [Google Scholar]
- Barth P. T., Datta N., Hedges R. W., Grinter N. J. Transposition of a deoxyribonucleic acid sequence encoding trimethoprim and streptomycin resistances from R483 to other replicons. J Bacteriol. 1976 Mar;125(3):800–810. doi: 10.1128/jb.125.3.800-810.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Coughter J. P., Johnston J. L., Archer G. L. Characterization of a staphylococcal trimethoprim resistance gene and its product. Antimicrob Agents Chemother. 1987 Jul;31(7):1027–1032. doi: 10.1128/aac.31.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bene V. E., John J. F., Jr, Twitty J. A., Lewis J. W. Anti-staphylococcal activity of teicoplanin, vancomycin, and other antimicrobial agents: the significance of methicillin resistance. J Infect Dis. 1986 Aug;154(2):349–352. doi: 10.1093/infdis/154.2.349. [DOI] [PubMed] [Google Scholar]
- Fling M. E., Walton L., Elwell L. P. Monitoring of plasmid-encoded, trimethoprim-resistant dihydrofolate reductase genes: detection of a new resistant enzyme. Antimicrob Agents Chemother. 1982 Nov;22(5):882–888. doi: 10.1128/aac.22.5.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein F. W., Papadopoulou B., Acar J. F. The changing pattern of trimethoprim resistance in Paris, with a review of worldwide experience. Rev Infect Dis. 1986 Sep-Oct;8(5):725–737. doi: 10.1093/clinids/8.5.725. [DOI] [PubMed] [Google Scholar]
- Hamilton-Miller J. M., Iliffe A. Antimicrobial resistance in coagulase-negative staphylococci. J Med Microbiol. 1985 Apr;19(2):217–226. doi: 10.1099/00222615-19-2-217. [DOI] [PubMed] [Google Scholar]
- Kraft C. A., Timbury M. C., Platt D. J. Distribution and genetic location of Tn7 in trimethoprim-resistant Escherichia coli. J Med Microbiol. 1986 Sep;22(2):125–131. doi: 10.1099/00222615-22-2-125. [DOI] [PubMed] [Google Scholar]
- Levitz R. E., Quintiliani R. Trimethoprim-sulfamethoxazole for bacterial meningitis. Ann Intern Med. 1984 Jun;100(6):881–890. doi: 10.7326/0003-4819-100-6-881. [DOI] [PubMed] [Google Scholar]
- Lewis E. L., Lacey R. W. Present significance of resistance to trimethoprim and sulphonamides in coliforms, Staphylococcus aureus, and Streptococcus faecalis. J Clin Pathol. 1973 Mar;26(3):175–180. doi: 10.1136/jcp.26.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon B. R., Skurray R. Antimicrobial resistance of Staphylococcus aureus: genetic basis. Microbiol Rev. 1987 Mar;51(1):88–134. doi: 10.1128/mr.51.1.88-134.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell R. W., Sweeney H. M., Cohen S. Conjugational transfer of gentamicin resistance plasmids intra- and interspecifically in Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 1983 Jan;23(1):151–160. doi: 10.1128/aac.23.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhla L. S. Resistance of Staphylococcus aureus to sulphamethoxazole and trimethoprim. J Clin Pathol. 1972 Aug;25(8):708–712. doi: 10.1136/jcp.25.8.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967 Sep;33(1):155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- Richardson J. F. Frequency of resistance to trimethoprim among isolates of Staphylococcus epidermidis and Staphylococcus saprophyticus. J Antimicrob Chemother. 1983 Feb;11(2):163–167. doi: 10.1093/jac/11.2.163. [DOI] [PubMed] [Google Scholar]
- Shapiro J. A., Sporn P. Tn402: a new transposable element determining trimethoprim resistance that inserts in bacteriophage lambda. J Bacteriol. 1977 Mar;129(3):1632–1635. doi: 10.1128/jb.129.3.1632-1635.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Towner K. J. A clinical isolate of Escherichia coli owing its trimethoprim resistance to a chromosomally-located trimethoprim transposon. J Antimicrob Chemother. 1981 Feb;7(2):157–162. doi: 10.1093/jac/7.2.157. [DOI] [PubMed] [Google Scholar]
- Ward T. T., Winn R. E., Hartstein A. I., Sewell D. L. Observations relating to an inter-hospital outbreak of methicillin-resistant Staphylococcus aureus: role of antimicrobial therapy in infection control. Infect Control. 1981 Nov-Dec;2(6):453–459. doi: 10.1017/s0195941700055715. [DOI] [PubMed] [Google Scholar]
- Weinstein R. A., Kabins S. A., Nathan C., Sweeney H. M., Jaffe H. W., Cohen S. Gentamicin-resistant staphylococci as hospital flora: epidemiology and resistance plasmids. J Infect Dis. 1982 Mar;145(3):374–382. doi: 10.1093/infdis/145.3.374. [DOI] [PubMed] [Google Scholar]
- Winter R. B., Gold L. Overproduction of bacteriophage Q beta maturation (A2) protein leads to cell lysis. Cell. 1983 Jul;33(3):877–885. doi: 10.1016/0092-8674(83)90030-2. [DOI] [PubMed] [Google Scholar]