Abstract
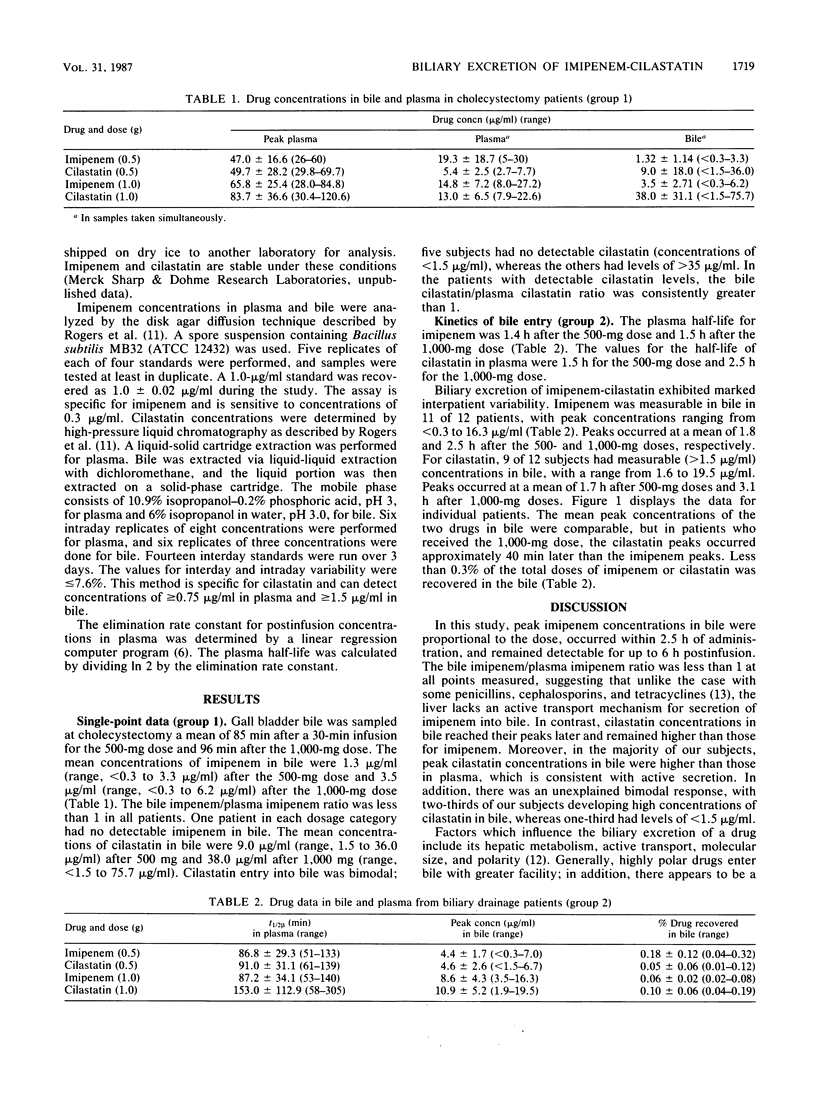
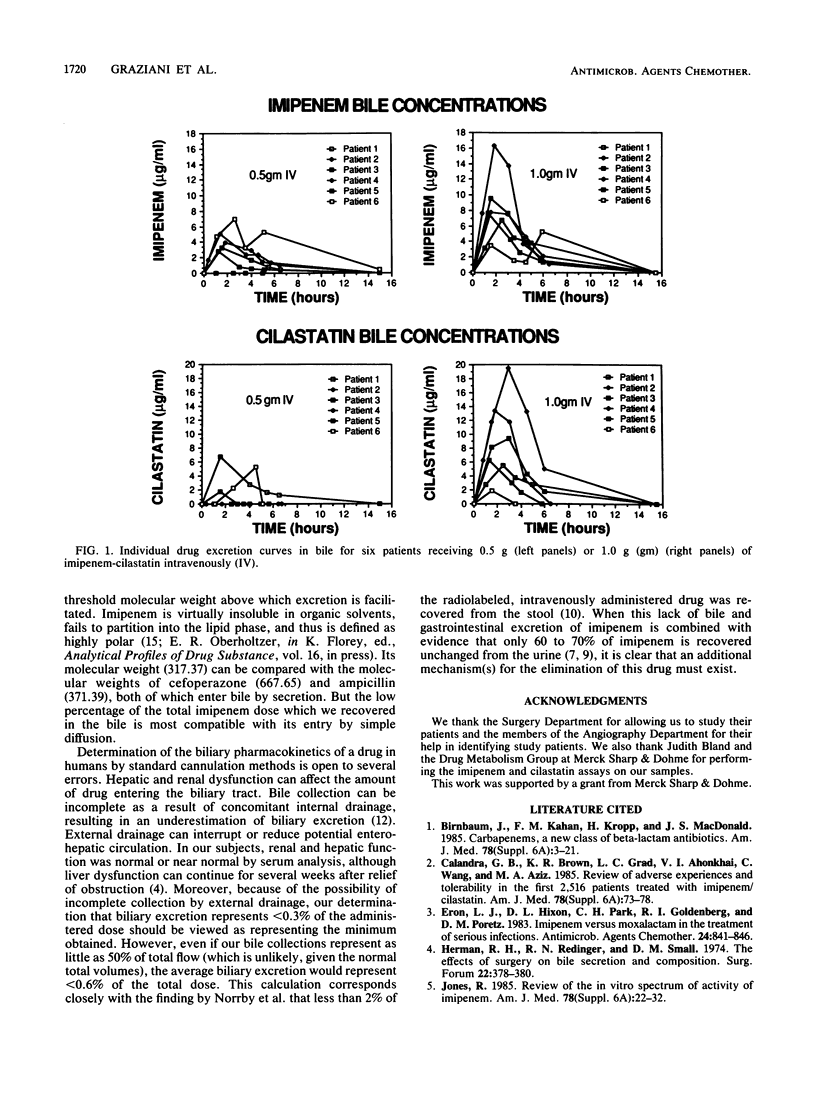
Imipenem-cilastatin concentrations in bile were measured in 12 cholecystectomy patients (group 1) and 12 patients with common duct drainage (group 2). Six patients in each group received 0.5 g, and six received 1.0 g intravenously over 30 to 60 min. In group 1, bile was collected a mean of 85 min postinfusion. The mean concentrations of imipenem in bile were 1.3 microgram/ml after the 0.5-g dose and 3.5 micrograms/ml after the 1.0-g dose. The mean concentrations of cilastatin in bile were 9.0 micrograms/ml after the 0.5-g dose and 38.0 micrograms/ml after the 1.0-g dose. In patients with common duct drainage, bile was collected predose and 0 to 2, 2 to 3, 3 to 4, and 4 to 6 h postinfusion. Peak imipenem concentrations in bile were 4.4 micrograms/ml after the 0.5-g dose and 8.6 micrograms/ml after the 1.0-g dose. Peak cilastatin concentrations in bile were 4.6 micrograms/ml for the 0.5-g dose and 10.9 micrograms/ml for the 1.0-g dose. Peak imipenem concentrations in bile occurred a mean of 2.3 h after administration of the drug; cilastatin peak concentrations occurred at a mean of 2.4 h. Less than 0.3% of each drug was recovered in the bile. Our results suggest that imipenem enters bile by simple diffusion and in most patients attains concentrations sufficient to inhibit susceptible organisms. In contrast, cilastatin had a bimodal entry into bile. Some patients had very high concentrations in bile, whereas others had very low or undetectable concentrations, suggesting that cilastatin may be actively secreted into the bile.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaum J., Kahan F. M., Kropp H., MacDonald J. S. Carbapenems, a new class of beta-lactam antibiotics. Discovery and development of imipenem/cilastatin. Am J Med. 1985 Jun 7;78(6A):3–21. doi: 10.1016/0002-9343(85)90097-x. [DOI] [PubMed] [Google Scholar]
- Calandra G. B., Brown K. R., Grad L. C., Ahonkhai V. I., Wang C., Aziz M. A. Review of adverse experiences and tolerability in the first 2,516 patients treated with imipenem/cilastatin. Am J Med. 1985 Jun 7;78(6A):73–78. doi: 10.1016/0002-9343(85)90104-4. [DOI] [PubMed] [Google Scholar]
- Eron L. J., Hixon D. L., Park C. H., Goldenberg R. I., Poretz D. M. Imipenem versus moxalactam in the treatment of serious infections. Antimicrob Agents Chemother. 1983 Dec;24(6):841–846. doi: 10.1128/aac.24.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman A. H., Redinger R. N., Small D. M., Egdahl R. H. The effects of surgery on bile secretion and composition. Surg Forum. 1971;22:378–380. [PubMed] [Google Scholar]
- Jones R. N. Review of the in vitro spectrum of activity of imipenem. Am J Med. 1985 Jun 7;78(6A):22–32. doi: 10.1016/0002-9343(85)90098-1. [DOI] [PubMed] [Google Scholar]
- Koeppe P., Hamann C. A program for non-linear regression analysis to be used on desk-top computers. Comput Programs Biomed. 1980 Dec;12(2-3):121–128. doi: 10.1016/0010-468x(80)90058-6. [DOI] [PubMed] [Google Scholar]
- MacGregor R. R., Gibson G. A., Bland J. A. Imipenem pharmacokinetics and body fluid concentrations in patients receiving high-dose treatment for serious infections. Antimicrob Agents Chemother. 1986 Feb;29(2):188–192. doi: 10.1128/aac.29.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nord C. E., Kager L., Philipson A., Stiernstedt G. Impact of imipenem/cilastatin therapy on faecal flora. Eur J Clin Microbiol. 1984 Oct;3(5):475–477. doi: 10.1007/BF02017379. [DOI] [PubMed] [Google Scholar]
- Norrby S. R., Alestig K., Björnegård B., Burman L. A., Ferber F., Huber J. L., Jones K. H., Kahan F. M., Kahan J. S., Kropp H. Urinary recovery of N-formimidoyl thienamycin (MK0787) as affected by coadministration of N-formimidoyl thienamycin dehydropeptidase inhibitors. Antimicrob Agents Chemother. 1983 Feb;23(2):300–307. doi: 10.1128/aac.23.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby S. R., Rogers J. D., Ferber F., Jones K. H., Zacchei A. G., Weidner L. L., Demetriades J. L., Gravallese D. A., Hsieh J. Y. Disposition of radiolabeled imipenem and cilastatin in normal human volunteers. Antimicrob Agents Chemother. 1984 Nov;26(5):707–714. doi: 10.1128/aac.26.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins D. E., Klaassen C. D. Biliary excretion of drugs in man. Clin Pharmacokinet. 1979 Sep-Oct;4(5):368–379. doi: 10.2165/00003088-197904050-00003. [DOI] [PubMed] [Google Scholar]
- Tally F. P., Jacobus N. V., Gorbach S. L. In vitro activity of N-formimidoyl thienamycin (MK0787). Antimicrob Agents Chemother. 1980 Oct;18(4):642–644. doi: 10.1128/aac.18.4.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Nikaido H. Diffusion of beta-lactam antibiotics through the porin channels of Escherichia coli K-12. Antimicrob Agents Chemother. 1985 Jan;27(1):84–92. doi: 10.1128/aac.27.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac B. A., Fisher M. A., Gibson G. A., MacGregor R. R. Safety and efficacy of high-dose treatment with imipenem-cilastatin in seriously ill patients. Antimicrob Agents Chemother. 1985 May;27(5):745–748. doi: 10.1128/aac.27.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]


