Abstract
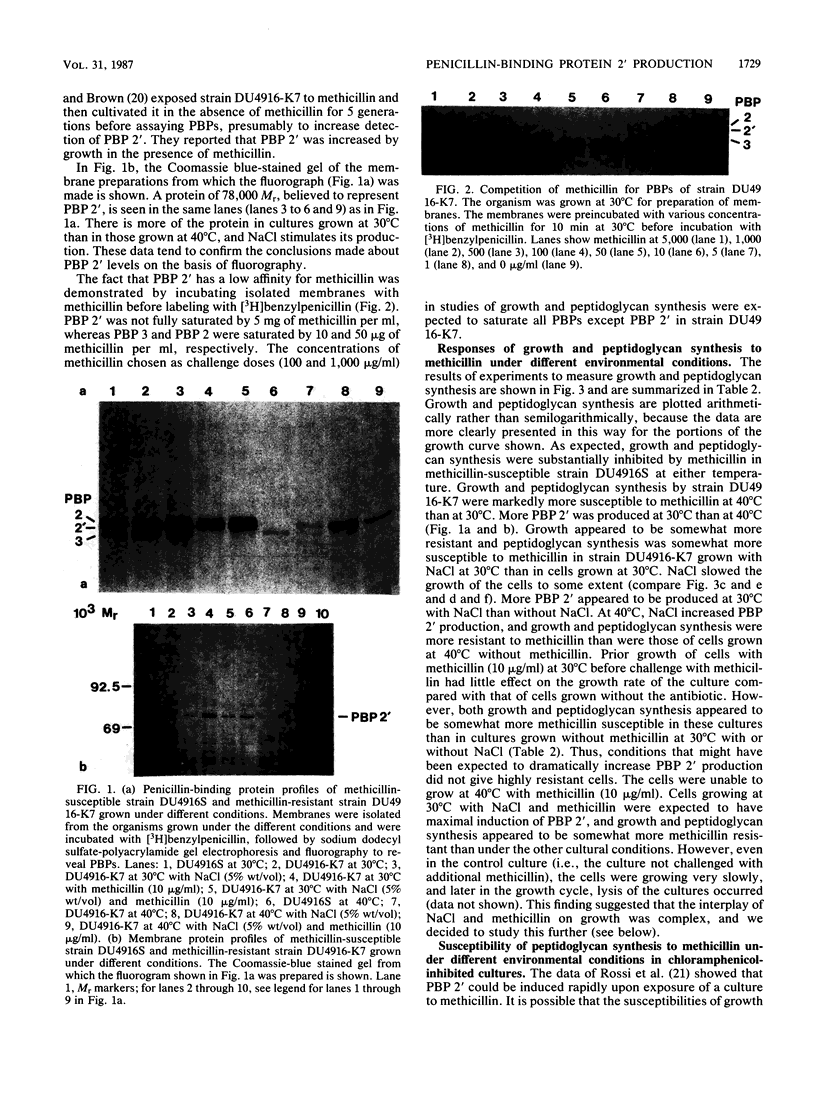
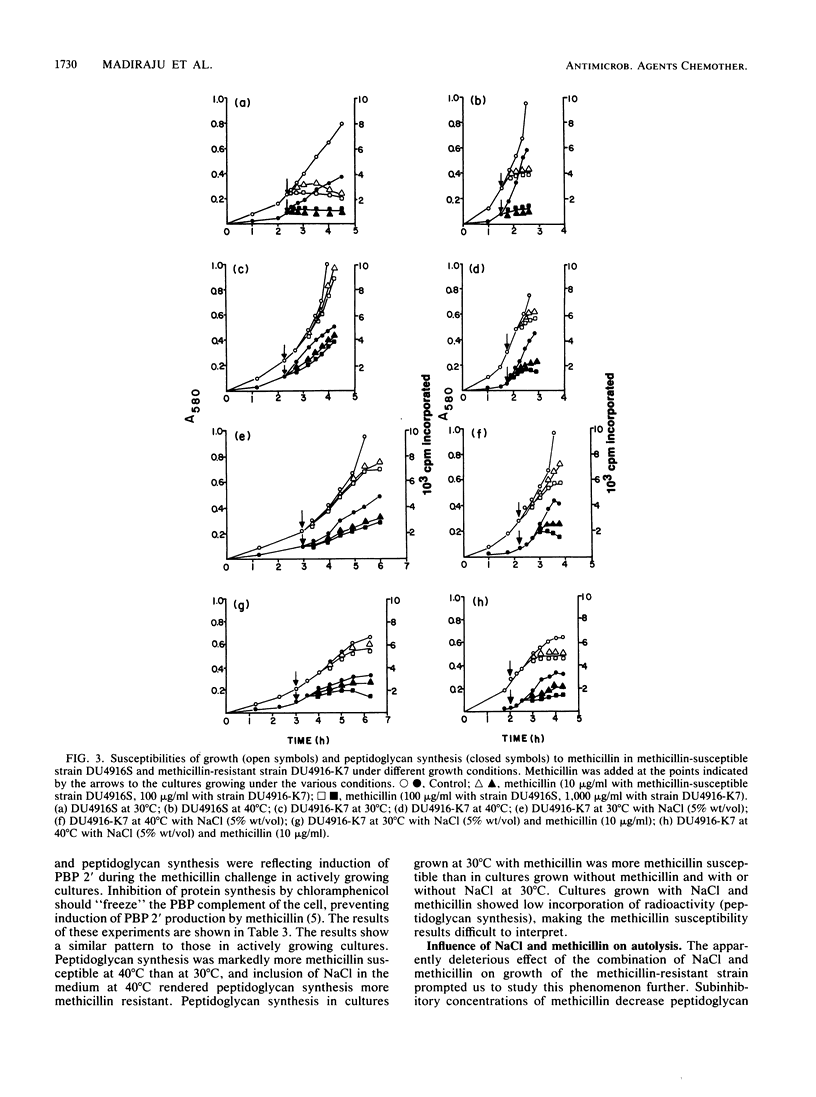
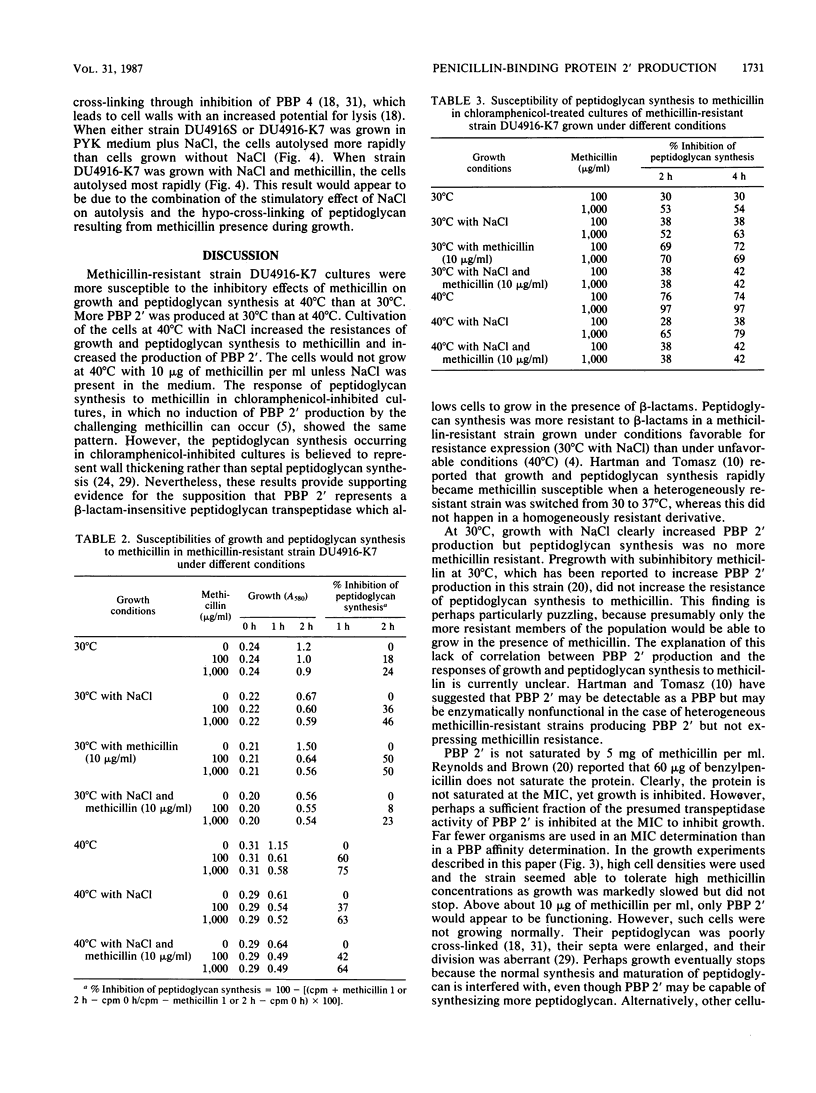
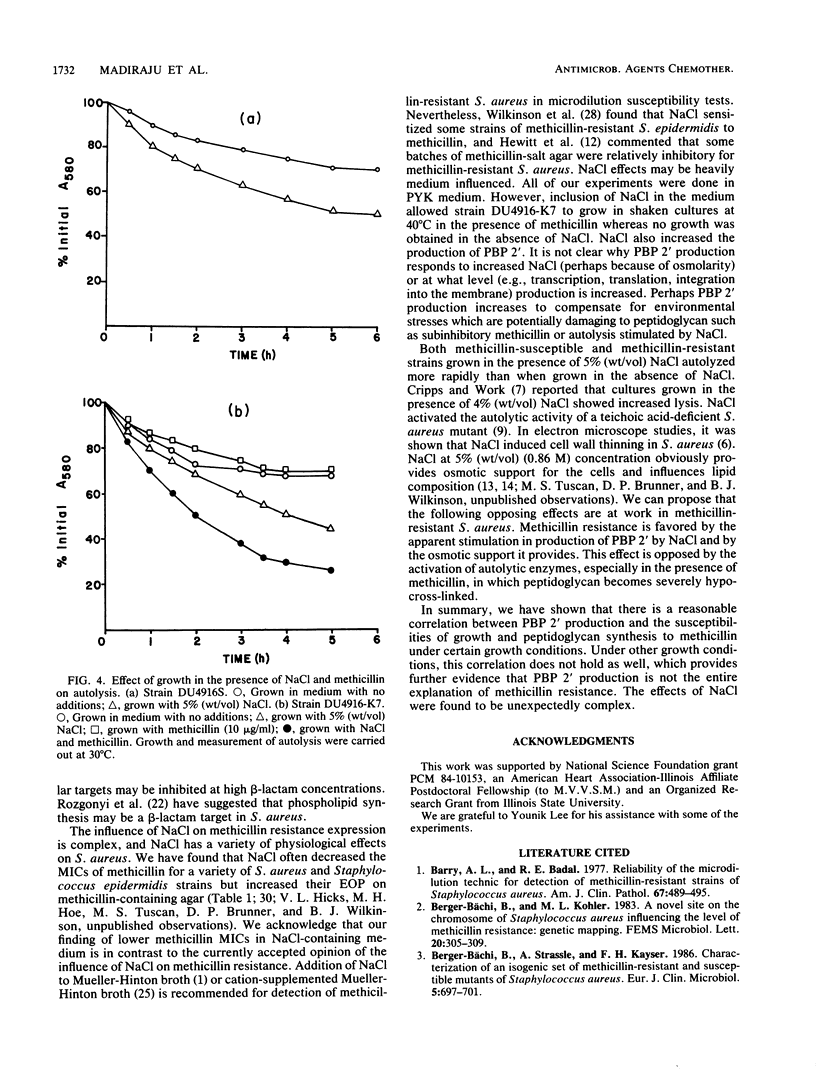
Methicillin-resistant Staphylococcus aureus strains produce a fifth penicillin-binding protein (PBP), PBP 2', with low affinity for beta-lactam antibiotics that is believed to represent a beta-lactam-insensitive peptidoglycan transpeptidase. In an effort to evaluate the adequacy of PBP 2' as an explanation of methicillin resistance, PBP 2' production and the responses of growth and peptidoglycan synthesis to methicillin under different environmental conditions have been compared. In the heterogeneous methicillin-resistant strain DU4916-K7, less PBP 2' was produced at 40 degrees C than at 30 degrees C, but inclusion of 5% (wt/vol) NaCl in the medium at 40 degrees C boosted PBP 2' production and allowed growth of the organism in the presence of 10 micrograms of methicillin per ml. When exponential-phase cultures were challenged with methicillin, growth and peptidoglycan synthesis were much more resistant at 30 degrees C than at 40 degrees C. Inclusion of NaCl in medium rendered growth and peptidoglycan synthesis more methicillin resistant at 40 degrees C. Hence, there was a good correlation between PBP 2' production and methicillin-resistant peptidoglycan synthesis under these conditions. However, PBP 2' production was increased by NaCl at 30 degrees C without markedly affecting the susceptibilities of growth and peptidoglycan synthesis to methicillin. Pregrowth of cells with methicillin, which was expected to boost PBP 2' production, seemed to increase the susceptibilities of growth and peptidoglycan synthesis to methicillin. Patterns of growth and peptidoglycan synthesis susceptibilities to methicillin which were similar to those described above were found in chloramphenicol-inhibited cultures, in which presumably no induction of PBP 2' could occur during the methicillin challenge period. Complex effects were noted in the combination of subinhibitory methicillin and NaCl. Growth of cells in the presence of NaCl stimulated their autolytic activity, which was further increased by growth with subinhibitory methicillin in addition to NaCl. It appears that NaCl enhances methicillin resistance by stimulating PBP 2' production and providing osmotic support but opposes it by stimulating autolytic activity which is exacerbated by the very low cross-linking of peptidoglycan in methicillin-resistant strains grown in the presence of methicillin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry A. L., Badal R. E. Reliability of the microdilution technic for detection of methicillin-resistant strains of staphylococcus aureus. Am J Clin Pathol. 1977 May;67(5):489–495. doi: 10.1093/ajcp/67.5.489. [DOI] [PubMed] [Google Scholar]
- Berger-Bächi B., Strässle A., Kayser F. H. Characterization of an isogenic set of methicillin-resistant and susceptible mutants of Staphylococcus aureus. Eur J Clin Microbiol. 1986 Dec;5(6):697–701. doi: 10.1007/BF02013308. [DOI] [PubMed] [Google Scholar]
- Chambers H. F., Hartman B. J., Tomasz A. Increased amounts of a novel penicillin-binding protein in a strain of methicillin-resistant Staphylococcus aureus exposed to nafcillin. J Clin Invest. 1985 Jul;76(1):325–331. doi: 10.1172/JCI111965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. M., Chatterjee A. N., Gilpin R. W., Young F. E. Ultrastructure of teichoic acid-deficient and other mutants of staphylococci. Ann N Y Acad Sci. 1974 Jul 31;236(0):22–53. doi: 10.1111/j.1749-6632.1974.tb41480.x. [DOI] [PubMed] [Google Scholar]
- Cripps R. E., Work E. The accumulation of extracellular macromolecules by Staphylococcus aureus grown in the presence of sodium chloride and glucose. J Gen Microbiol. 1967 Oct;49(1):127–137. doi: 10.1099/00221287-49-1-127. [DOI] [PubMed] [Google Scholar]
- Dornbusch K., Hallander H. O. Transduction of penicillinase production and methicillin resistance-enterotoxin B production in strains of Staphylococcus aureus. J Gen Microbiol. 1973 May;76(1):1–11. doi: 10.1099/00221287-76-1-1. [DOI] [PubMed] [Google Scholar]
- Gilpin R. W., Chatterjee A. N., Young F. E. Autolysis of microbial cells: salt activation of autolytic enzymes in a mutant of Staphylococcus aureus. J Bacteriol. 1972 Jul;111(1):272–283. doi: 10.1128/jb.111.1.272-283.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Expression of methicillin resistance in heterogeneous strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Jan;29(1):85–92. doi: 10.1128/aac.29.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984 May;158(2):513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt J. H., Coe A. W., Parker M. T. The detection of methicillin resistance in Staphylococcus aureus. J Med Microbiol. 1969 Nov 4;2(4):443–456. doi: 10.1099/00222615-2-4-443. [DOI] [PubMed] [Google Scholar]
- Hurst A., Ofori E., Vishnubhatla I., Kates M. Adaptational changes in Staphylococcus aureus MF 31 grown above its maximum growth temperature when protected by sodium chloride: lipid studies. Can J Microbiol. 1984 Nov;30(11):1424–1427. doi: 10.1139/m84-227. [DOI] [PubMed] [Google Scholar]
- Kanemasa Y., Yoshioka T., Hayashi H. Alteration of the phospholipid composition of Staphylococcus aureus cultured in medium containing NaCl. Biochim Biophys Acta. 1972 Nov 30;280(3):444–450. [PubMed] [Google Scholar]
- Lacey R. W. Staphylococcus aureus strain DU4916--an atypical methicillin-resistant isolate? J Gen Microbiol. 1974 Sep;84(1):1–10. doi: 10.1099/00221287-84-1-1. [DOI] [PubMed] [Google Scholar]
- Matsuhashi M., Song M. D., Ishino F., Wachi M., Doi M., Inoue M., Ubukata K., Yamashita N., Konno M. Molecular cloning of the gene of a penicillin-binding protein supposed to cause high resistance to beta-lactam antibiotics in Staphylococcus aureus. J Bacteriol. 1986 Sep;167(3):975–980. doi: 10.1128/jb.167.3.975-980.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mychajlonka M., McDowell T. D., Shockman G. D. Conservation of cell wall peptidoglycan by strains of Streptococcus mutans and Streptococcus sanguis. Infect Immun. 1980 Apr;28(1):65–73. doi: 10.1128/iai.28.1.65-73.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qoronfleh M. W., Wilkinson B. J. Effects of growth of methicillin-resistant and -susceptible Staphylococcus aureus in the presence of beta-lactams on peptidoglycan structure and susceptibility to lytic enzymes. Antimicrob Agents Chemother. 1986 Feb;29(2):250–257. doi: 10.1128/aac.29.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P. E., Brown D. F. Penicillin-binding proteins of beta-lactam-resistant strains of Staphylococcus aureus. Effect of growth conditions. FEBS Lett. 1985 Nov 11;192(1):28–32. doi: 10.1016/0014-5793(85)80036-3. [DOI] [PubMed] [Google Scholar]
- Reynolds P. E. Resistance of the antibiotic target site. Br Med Bull. 1984 Jan;40(1):3–10. doi: 10.1093/oxfordjournals.bmb.a071944. [DOI] [PubMed] [Google Scholar]
- Rossi L., Tonin E., Cheng Y. R., Fontana R. Regulation of penicillin-binding protein activity: description of a methicillin-inducible penicillin-binding protein in Staphylococcus aureus. Antimicrob Agents Chemother. 1985 May;27(5):828–831. doi: 10.1128/aac.27.5.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozgonyi F., Kiss J., Jékel P., Váczi L. Effect of methicillin on the phospholipid content of methicillin sensitive Staphylococcus aureus. Acta Microbiol Acad Sci Hung. 1980;27(1):31–40. [PubMed] [Google Scholar]
- Sabath L. D. Chemical and physical factors influencing methicillin resistance of Staphylococcus aureus and Staphylococcus epidermidis. J Antimicrob Chemother. 1977 Nov;3 (Suppl 100):47–51. doi: 10.1093/jac/3.suppl_c.47. [DOI] [PubMed] [Google Scholar]
- Smith P. F., Wilkinson B. J. Differential methicillin susceptibilities of peptidoglycan syntheses in methicillin-resistant Staphylococcus aureus. J Bacteriol. 1981 Nov;148(2):610–617. doi: 10.1128/jb.148.2.610-617.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C., McDougal L. K. Successful use of broth microdilution in susceptibility tests for methicillin-resistant (heteroresistant) staphylococci. J Clin Microbiol. 1983 Nov;18(5):1084–1091. doi: 10.1128/jcm.18.5.1084-1091.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubukata K., Yamashita N., Konno M. Occurrence of a beta-lactam-inducible penicillin-binding protein in methicillin-resistant staphylococci. Antimicrob Agents Chemother. 1985 May;27(5):851–857. doi: 10.1128/aac.27.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsui Y., Yokota T. Role of an altered penicillin-binding protein in methicillin- and cephem-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1985 Sep;28(3):397–403. doi: 10.1128/aac.28.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Maxwell S., Schaus S. M. Classification and characteristics of coagulase-negative, methicillin-resistant staphylococci. J Clin Microbiol. 1980 Aug;12(2):161–166. doi: 10.1128/jcm.12.2.161-166.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Nadakavukaren M. J. Methicillin-resistant septal peptidoglycan synthesis in a methicillin-resistant Staphylococcus aureus strain. Antimicrob Agents Chemother. 1983 Apr;23(4):610–613. doi: 10.1128/aac.23.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyke A. W., Ward J. B., Hayes M. V. Synthesis of peptidoglycan in vivo in methicillin-resistant Staphylococcus aureus. Eur J Biochem. 1982 Oct;127(3):553–558. doi: 10.1111/j.1432-1033.1982.tb06907.x. [DOI] [PubMed] [Google Scholar]