Abstract
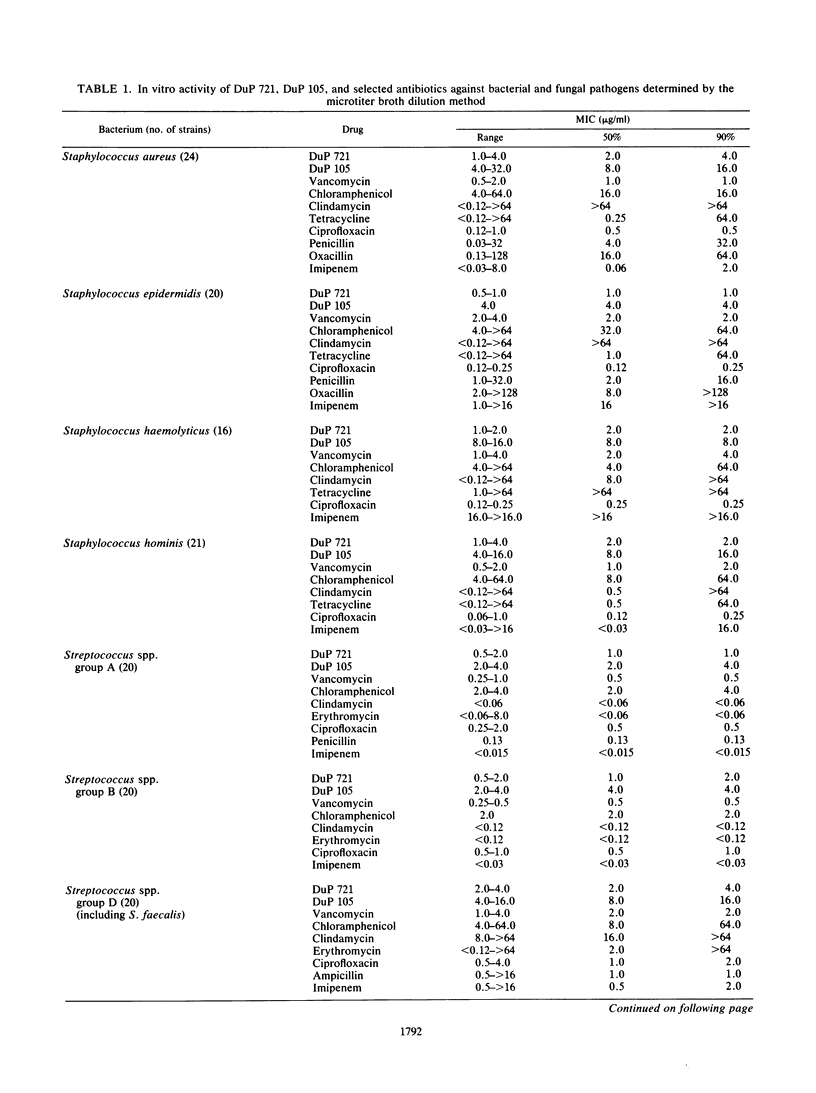
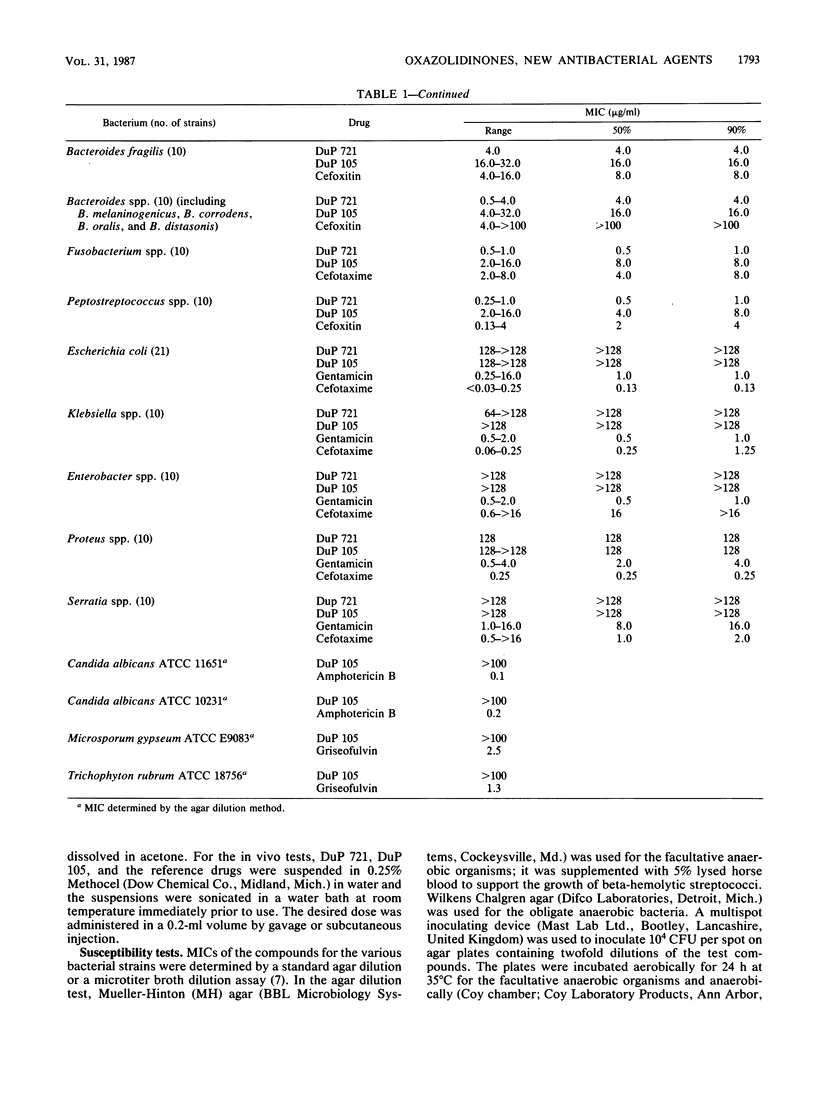
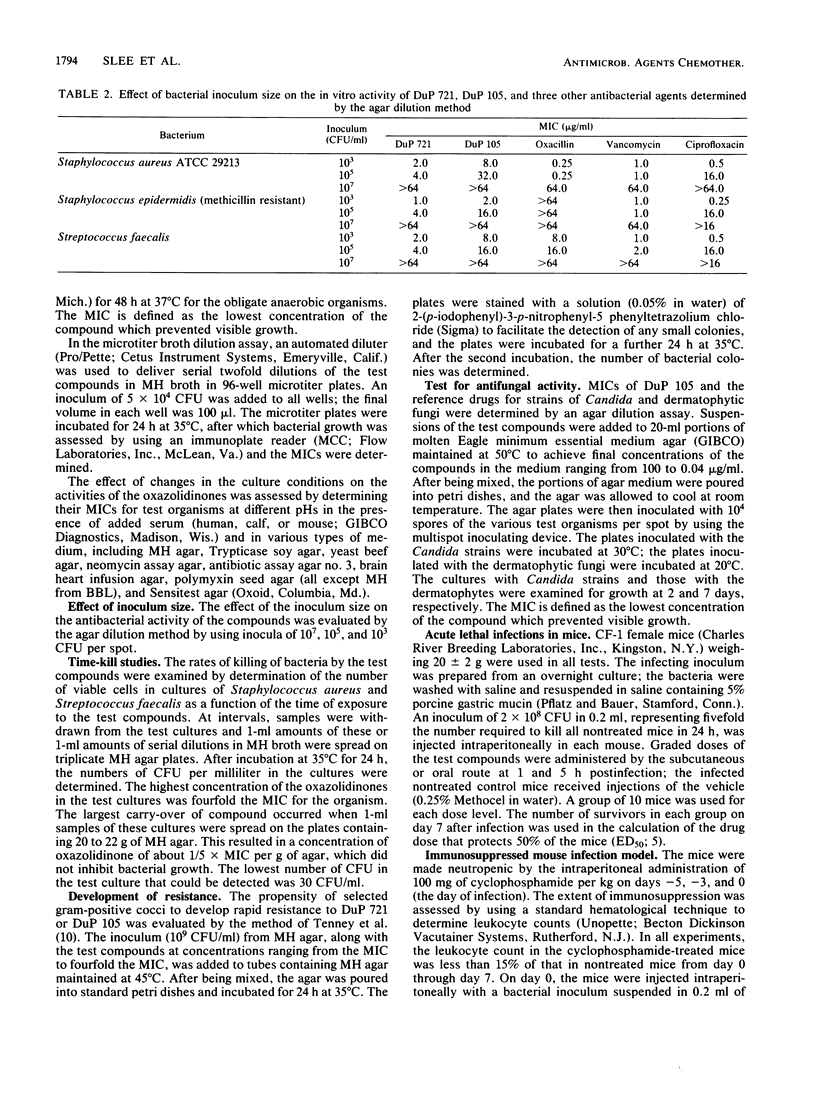
DuP 721 (p-acetylphenyloxooxazolidinylmethylacetamide) and DuP 105 (a methylsulfinyl derivative) are orally active representatives of the oxazolidinones, a new class of synthetic antibacterial agents. Their antibacterial spectrum includes staphylococci, streptococci, and Bacteroides fragilis strains. The compounds have equal activity against staphylococcal strains susceptible or resistant to beta-lactam antibiotics, including methicillin-resistant strains. The MICs for 90% of the strains (MIC90s) against staphylococcal isolates were 1 to 4 micrograms/ml for DuP 721 and 4 to 16 micrograms/ml for DuP 105, compared with 1 to 2 micrograms/ml for vancomycin, 0.5 microgram/ml for ciprofloxacin, and 2 to greater than 16 micrograms/ml for imipenem. The MIC90s against group D streptococci were 4 micrograms/ml for DuP 721, 16 micrograms/ml for DuP 105, and 2 micrograms/ml for vancomycin, ciprofloxacin, and imipenem. MIC90s against B. fragilis isolates were 4 micrograms/ml for DuP 721, 16 micrograms/ml for DuP 105, and 8 micrograms/ml for cefoxitin. DuP 721 and DuP 105 administered by either the oral or the parenteral route were protective against staphylococcal and streptococcal infections in mice. The 50% effective doses were 2 to 10 mg/kg for DuP 721, 9 to 23 mg/kg for DuP 105, and 2 to 12 mg/kg for vancomycin. These results indicate that further studies of compounds of the oxazolidinone series are warranted.
Full text
PDF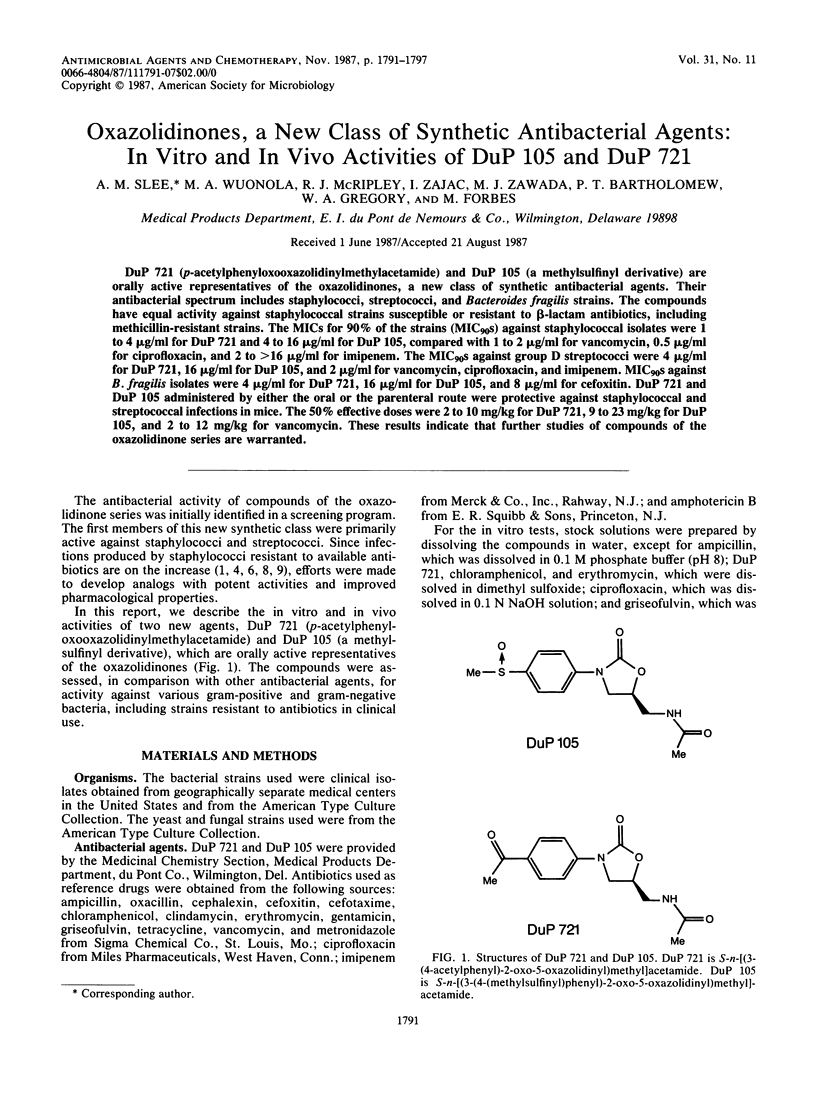
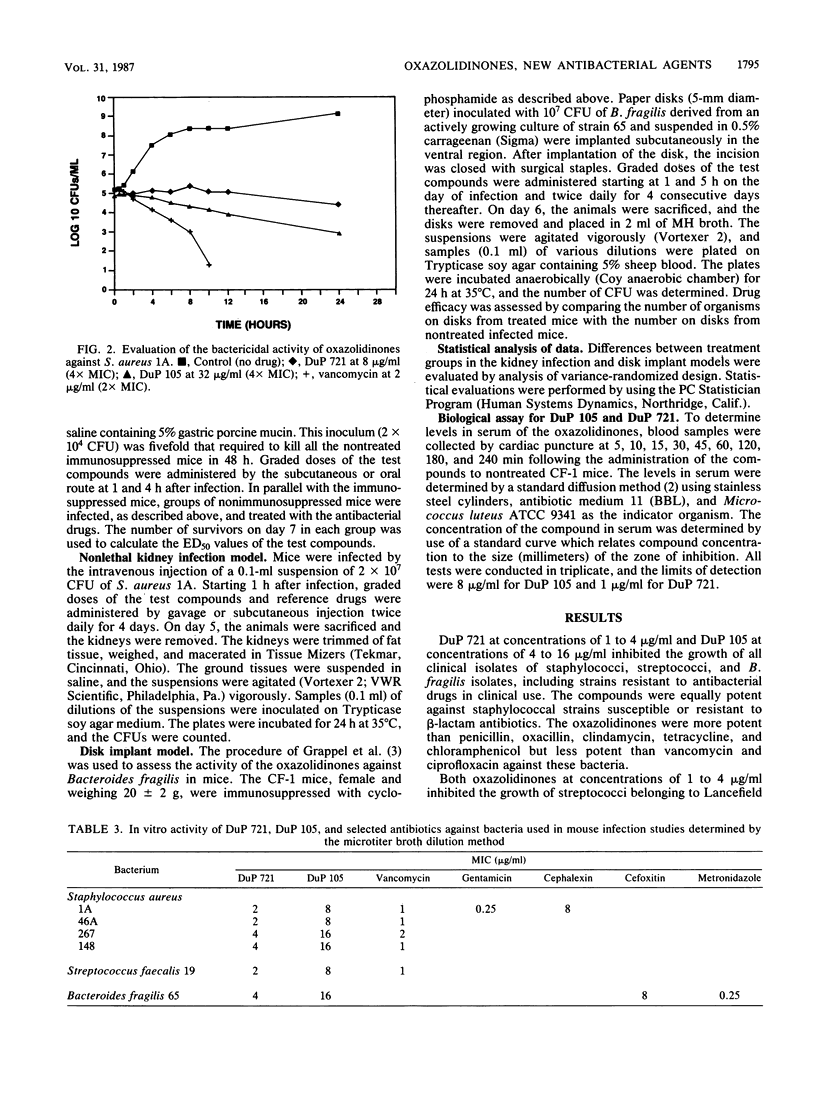
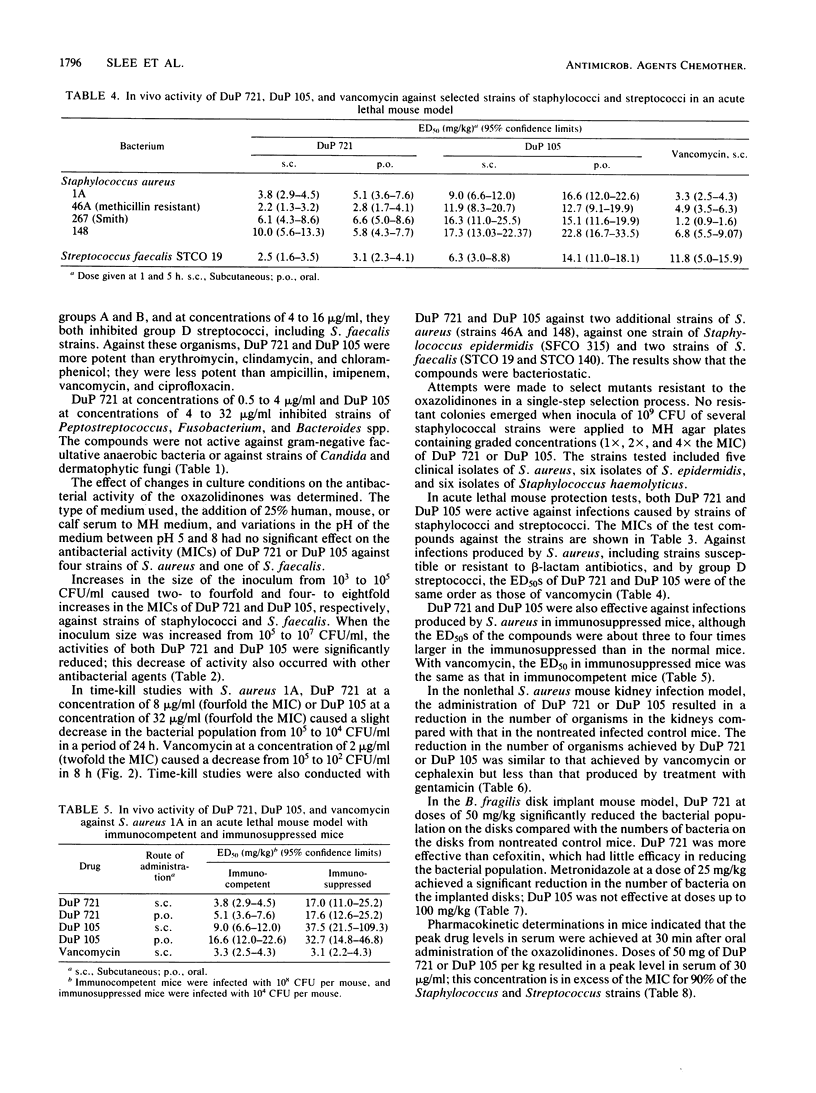
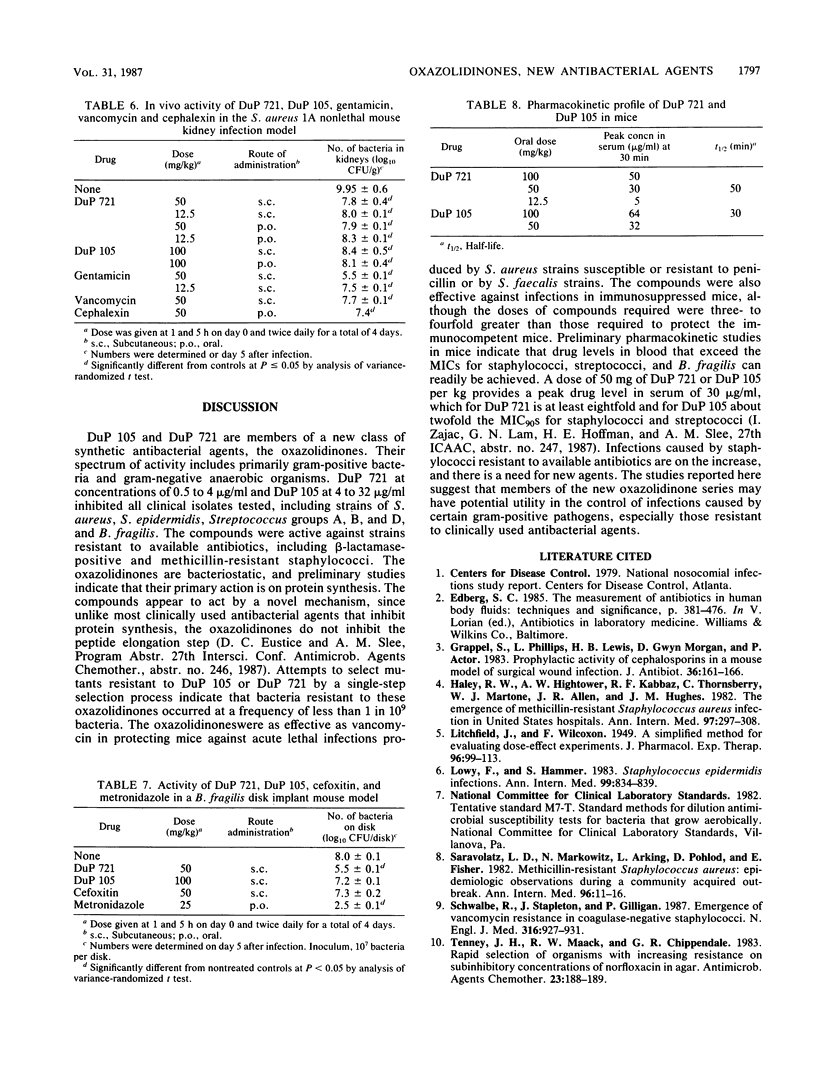
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Grappel S. F., Phillips L., Lewis H. B., Morgan D. G., Actor P. Prophylactic activity of cephalosporins in a mouse model of surgical wound infection. J Antibiot (Tokyo) 1983 Feb;36(2):161–166. doi: 10.7164/antibiotics.36.161. [DOI] [PubMed] [Google Scholar]
- Haley R. W., Hightower A. W., Khabbaz R. F., Thornsberry C., Martone W. J., Allen J. R., Hughes J. M. The emergence of methicillin-resistant Staphylococcus aureus infections in United States hospitals. Possible role of the house staff-patient transfer circuit. Ann Intern Med. 1982 Sep;97(3):297–308. doi: 10.7326/0003-4819-97-3-297. [DOI] [PubMed] [Google Scholar]
- Lowy F. D., Hammer S. M. Staphylococcus epidermidis infections. Ann Intern Med. 1983 Dec;99(6):834–839. doi: 10.7326/0003-4819-99-6-834. [DOI] [PubMed] [Google Scholar]
- Saravolatz L. D., Markowitz N., Arking L., Pohlod D., Fisher E. Methicillin-resistant Staphylococcus aureus. Epidemiologic observations during a community-acquired outbreak. Ann Intern Med. 1982 Jan;96(1):11–16. doi: 10.7326/0003-4819-96-1-11. [DOI] [PubMed] [Google Scholar]
- Schwalbe R. S., Stapleton J. T., Gilligan P. H. Emergence of vancomycin resistance in coagulase-negative staphylococci. N Engl J Med. 1987 Apr 9;316(15):927–931. doi: 10.1056/NEJM198704093161507. [DOI] [PubMed] [Google Scholar]
- Tenney J. H., Maack R. W., Chippendale G. R. Rapid selection of organisms with increasing resistance on subinhibitory concentrations of norfloxacin in agar. Antimicrob Agents Chemother. 1983 Jan;23(1):188–189. doi: 10.1128/aac.23.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]



