Abstract
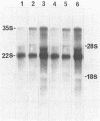
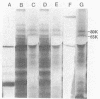
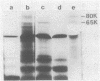
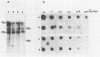
The mode of inhibition of N-methylisatin-beta-4',4'-diethylthiosemicarbazone (M-IBDET) on Moloney leukemia virus production was studied. Drug treatment of infected cells did not alter the amounts or sizes of the 35S and 22S subgenomic viral RNAs. The translation abilities of poly(A)+ RNA derived from M-IBDET-treated cells was also unaffected, as judged by cell-free translation analysis. Poly(A)+ RNA derived from M-IBDET-treated cells directed translation of equal amounts of viral gag precursors, gPr-80gag and Pr-65gag, as did poly(A)+ RNA prepared from untreated cells. The addition of M-IBDET to a cell-free translation system programmed with either total poly(A)+ RNA extracted from infected cells or hybrid-selected viral RNA inhibited the synthesis of viral protein precursors. An examination of the effect of M-IBDET on polysomes engaged in the translation of viral proteins revealed a fourfold accumulation of polysomal virus-specific RNA in drug-treated cells. These results suggest that the inhibition of Moloney leukemia virus by M-IBDET involves a block in the translation of viral RNA rather than interference with viral RNA transcription.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacheler L., Fan H. Isolation of recombinant DNA clones carrying complete integrated proviruses of Moloney murine leukemia virus. J Virol. 1981 Jan;37(1):181–190. doi: 10.1128/jvi.37.1.181-190.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer D. J. Clinical experience with the antiviral drug marboran (1-methylisatin 3-thiosemicarbazone). Ann N Y Acad Sci. 1965 Jul 30;130(1):110–117. doi: 10.1111/j.1749-6632.1965.tb12545.x. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Fan H., Verma I. M. Size analysis and relationship of murine leukemia virus-specific mRNA's: evidence for transposition of sequences during synthesis and processing of subgenomic mRNA. J Virol. 1978 May;26(2):468–478. doi: 10.1128/jvi.26.2.468-478.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellous M., Nir U., Wallach D., Merlin G., Rubinstein M., Revel M. Interferon-dependent induction of mRNA for the major histocompatibility antigens in human fibroblasts and lymphoblastoid cells. Proc Natl Acad Sci U S A. 1982 May;79(10):3082–3086. doi: 10.1073/pnas.79.10.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzburg I., de Baetselier A., Walker M. D., Behar L., Lehrach H., Frischauf A. M., Littauer U. Z. Brain tubulin and actin cDNA sequences: isolation of recombinant plasmids. Nucleic Acids Res. 1980 Aug 25;8(16):3553–3564. doi: 10.1093/nar/8.16.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E., Felix H. Synthesis of vaccinia viral antigens in HeLa cells in the presence of isatin beta-thiosemicarbazone. Antimicrob Agents Chemother. 1977 Feb;11(2):202–208. doi: 10.1128/aac.11.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ng V. L., Wood T. G., Lyons D. D., Arlinghaus R. B. Characterization of intracellular precursor polyproteins of Moloney murine leukemia virus. J Virol. 1979 Dec;32(3):1051–1056. doi: 10.1128/jvi.32.3.1051-1056.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G. D., Zimmerman E. F. Inhibition of poliovirus replication by N-methylisatin-beta-4':4'-dibutylthiosemicarbazone. Virology. 1969 Aug;38(4):641–650. doi: 10.1016/0042-6822(69)90183-4. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ronen D., Nir E., Teitz Y. Effect of N-methylisatin-beta-4':4'-diethylthiosemicarbazone on intracellular Moloney leukemia virus constituents. Antiviral Res. 1985 Aug;5(4):249–254. doi: 10.1016/0166-3542(85)90029-4. [DOI] [PubMed] [Google Scholar]
- Ronen D., Teitz Y. Inhibition of the synthesis of Moloney leukemia virus structural proteins by N-methylisatin-beta-4',4'-diethylthiosemicarbazone. Antimicrob Agents Chemother. 1984 Dec;26(6):913–916. doi: 10.1128/aac.26.6.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L., Dafni N., Lieman-Hurwitz J., Groner Y. Nucleotide sequence and expression of human chromosome 21-encoded superoxide dismutase mRNA. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5465–5469. doi: 10.1073/pnas.80.18.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L., Edelstein F., Shtacher G., Avramoff M., Teitz Y. Inhibition of Moloney leukaemia virus production by N-methylisatin-beta-4':4-diethylthiosemicarbazone. J Gen Virol. 1980 Jan;46(1):195–203. doi: 10.1099/0022-1317-46-1-195. [DOI] [PubMed] [Google Scholar]
- Sherman L., Teitz Y. Glycoprotein enrichment in Moloney leukemia virus structural proteins released from interferon-treated cells. Exp Cell Biol. 1981;49(4):185–194. doi: 10.1159/000163822. [DOI] [PubMed] [Google Scholar]
- Teitz Y., Lennette E. H., Oshiro L. S., Cremer N. E. Release of C-type particles from normal rat thymus cultures and those infected with Moloney leukemia virus. J Natl Cancer Inst. 1971 Jan;46(1):11–23. [PubMed] [Google Scholar]
- Woodson B., Joklik W. K. The inhibition of vaccinia virus multiplication by isatin-beta-thiosemicarbazone. Proc Natl Acad Sci U S A. 1965 Sep;54(3):946–953. doi: 10.1073/pnas.54.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaane D. V., Gielkens A. L., Hesselink W. G., Bloemers H. P. Identification of Rauscher murine leukemia virus-specific mRNAs for the synthesis of gag- and env-gene products. Proc Natl Acad Sci U S A. 1977 May;74(5):1855–1859. doi: 10.1073/pnas.74.5.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]