Abstract
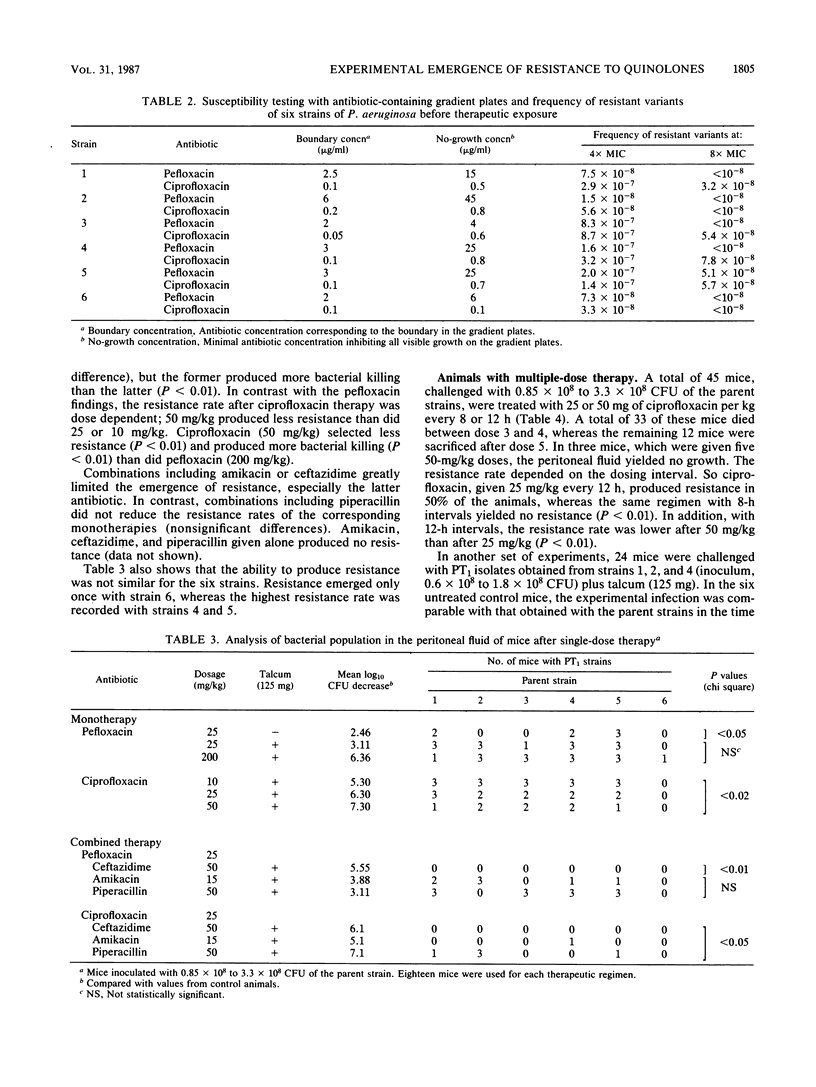
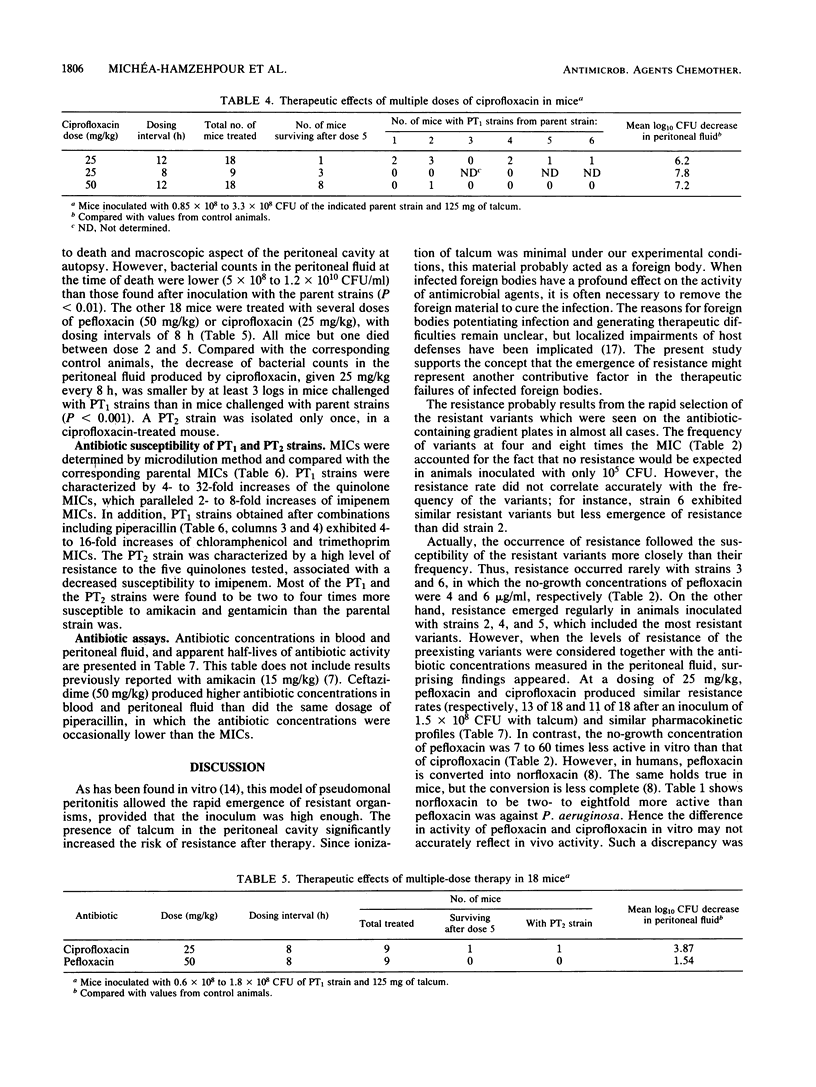
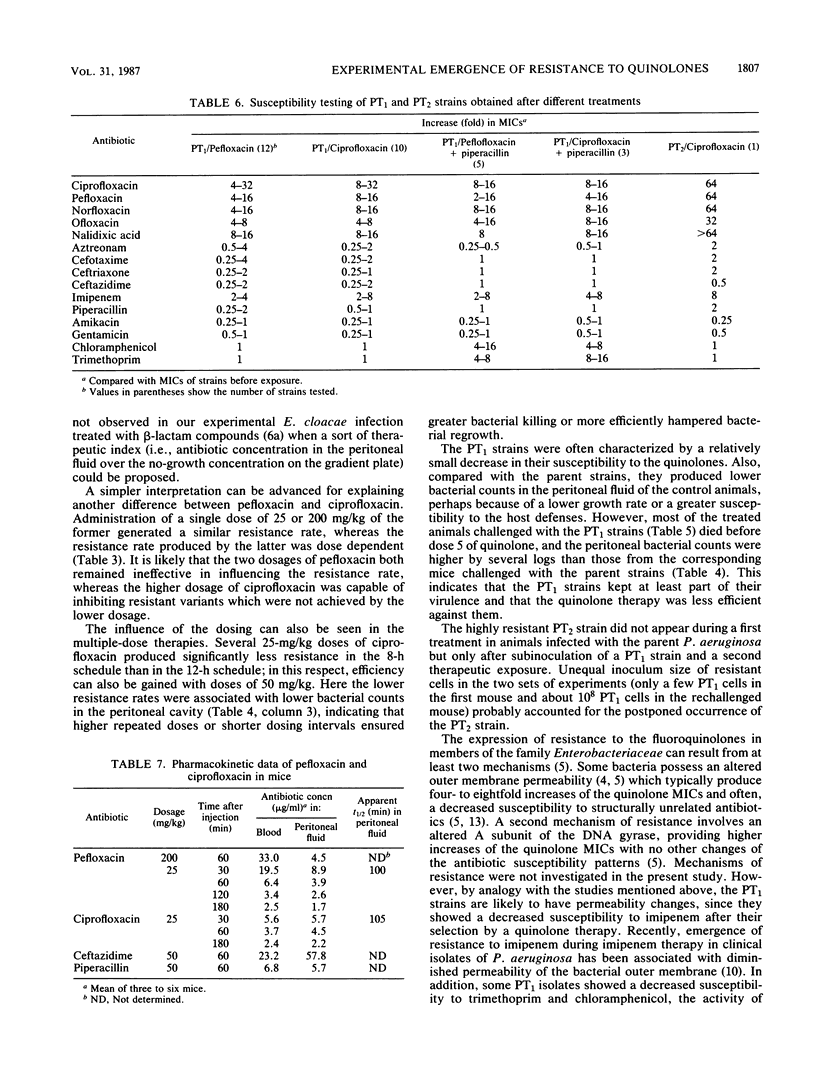
Resistance emerging after fluoroquinolone therapy was investigated in a murine model of Pseudomonas aeruginosa infection. Mice were infected intraperitoneally by one of six strains and treated with pefloxacin or ciprofloxacin. In mice challenged with a low inoculum (1.6 X 10(5) CFU), no resistance occurred. With a higher inoculum (1.5 X 10(8) CFU) and after a single dose of antibiotic, posttherapy (PT1) strains with decreased susceptibility to quinolones (4- to 32-fold less) were isolated at a variable rate. The presence of talcum (125 mg) in the peritoneal cavity increased the risk of resistance after therapy. Pefloxacin (25 or 200 mg/kg) and ciprofloxacin (25 mg/kg) yielded similar resistance rates (61 to 77%), but ciprofloxacin (10 mg/kg) produced more resistance (83%) than did ciprofloxacin (50 mg/kg) (44%) (P less than 0.02). Combined with a quinolone, ceftazidime (P less than 0.001) or amikacin (P less than 0.01), but not piperacillin, reduced the emergence of resistance. After several doses of ciprofloxacin, it was found that 25-mg/kg doses every 12 h produced more resistance than did 25-mg/kg doses every 8 h or 50-mg/kg doses every 12 h. Compared with the preceding experiments using parent strains, ciprofloxacin and pefloxacin were less efficient in killing bacteria in mice infected with PT1 strains. Moreover, in one of these mice, a highly resistant PT2 strain (64-fold MIC increase for the quinolones) emerged. Besides increased MICs of the quinolones, there was a two- to eightfold increase in imipenem MIC for all PT1 and PT2 strains without alteration of other beta-lactam and aminoglycoside susceptibility. Some PT1 strains also showed a decreased susceptibility to trimethoprim and chloramphenicol. During therapy with a quinolone, resistance can emerge rapidly, especially when there is a large number of bacteria or a foreign body present. This risk may depend on the dosing schedule and may be reduced by combined therapy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auckenthaler R., Michéa-Hamzehpour M., Pechère J. C. In-vitro activity of newer quinolones against aerobic bacteria. J Antimicrob Chemother. 1986 Apr;17 (Suppl B):29–39. doi: 10.1093/jac/17.suppl_b.29. [DOI] [PubMed] [Google Scholar]
- Bryson V., Szybalski W. Microbial Selection. Science. 1952 Jul 18;116(3003):45–51. doi: 10.1126/science.116.3003.45. [DOI] [PubMed] [Google Scholar]
- Gutmann L., Williamson R., Moreau N., Kitzis M. D., Collatz E., Acar J. F., Goldstein F. W. Cross-resistance to nalidixic acid, trimethoprim, and chloramphenicol associated with alterations in outer membrane proteins of Klebsiella, Enterobacter, and Serratia. J Infect Dis. 1985 Mar;151(3):501–507. doi: 10.1093/infdis/151.3.501. [DOI] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Irikura T., Iyobe S., Mitsuhashi S. Differences in susceptibility to quinolones of outer membrane mutants of Salmonella typhimurium and Escherichia coli. Antimicrob Agents Chemother. 1986 Mar;29(3):535–538. doi: 10.1128/aac.29.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Souza K. S., Tung C., McHugh G. L., Swartz M. N. Genetic and biochemical characterization of norfloxacin resistance in Escherichia coli. Antimicrob Agents Chemother. 1986 Apr;29(4):639–644. doi: 10.1128/aac.29.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A., Shannon K., Phillips I. The in-vitro activity of ciprofloxacin compared with that of norfloxacin and nalidixic acid. J Antimicrob Chemother. 1984 Apr;13(4):325–331. doi: 10.1093/jac/13.4.325. [DOI] [PubMed] [Google Scholar]
- Marchou B., Michea-Hamzehpour M., Lucain C., Pechère J. C. Development of beta-lactam-resistant Enterobacter cloacae in mice. J Infect Dis. 1987 Aug;156(2):369–373. doi: 10.1093/infdis/156.2.369. [DOI] [PubMed] [Google Scholar]
- Michéa-Hamzehpour M., Pechère J. C., Marchou B., Auckenthaler R. Combination therapy: a way to limit emergence of resistance? Am J Med. 1986 Jun 30;80(6B):138–142. doi: 10.1016/0002-9343(86)90491-2. [DOI] [PubMed] [Google Scholar]
- Montay G., Goueffon Y., Roquet F. Absorption, distribution, metabolic fate, and elimination of pefloxacin mesylate in mice, rats, dogs, monkeys, and humans. Antimicrob Agents Chemother. 1984 Apr;25(4):463–472. doi: 10.1128/aac.25.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechère J. C., Marchou B., Michéa-Hamzehpour M., Auckenthaler R. Emergence of resistance after therapy with antibiotics used alone or combined in a murine model. J Antimicrob Chemother. 1986 Mar;17 (Suppl A):11–18. doi: 10.1093/jac/17.suppl_a.11. [DOI] [PubMed] [Google Scholar]
- Quinn J. P., Dudek E. J., DiVincenzo C. A., Lucks D. A., Lerner S. A. Emergence of resistance to imipenem during therapy for Pseudomonas aeruginosa infections. J Infect Dis. 1986 Aug;154(2):289–294. doi: 10.1093/infdis/154.2.289. [DOI] [PubMed] [Google Scholar]
- Rubio T. T., Shapiro C. Ciprofloxacin in the treatment of Pseudomonas infection in cystic fibrosis patients. J Antimicrob Chemother. 1986 Nov;18 (Suppl 500):147–152. doi: 10.1093/jac/18.supplement_d.147. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Goering R. V., Werner V. Selection of multiple antibiotic resistance by quinolones, beta-lactams, and aminoglycosides with special reference to cross-resistance between unrelated drug classes. Antimicrob Agents Chemother. 1984 Dec;26(6):797–801. doi: 10.1128/aac.26.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Microbial resistance to newer generation beta-lactam antibiotics: clinical and laboratory implications. J Infect Dis. 1985 Mar;151(3):399–406. doi: 10.1093/infdis/151.3.399. [DOI] [PubMed] [Google Scholar]
- Tenney J. H., Maack R. W., Chippendale G. R. Rapid selection of organisms with increasing resistance on subinhibitory concentrations of norfloxacin in agar. Antimicrob Agents Chemother. 1983 Jan;23(1):188–189. doi: 10.1128/aac.23.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli W., Waldvogel F. A., Vaudaux P., Nydegger U. E. Pathogenesis of foreign body infection: description and characteristics of an animal model. J Infect Dis. 1982 Oct;146(4):487–497. doi: 10.1093/infdis/146.4.487. [DOI] [PubMed] [Google Scholar]


