Abstract
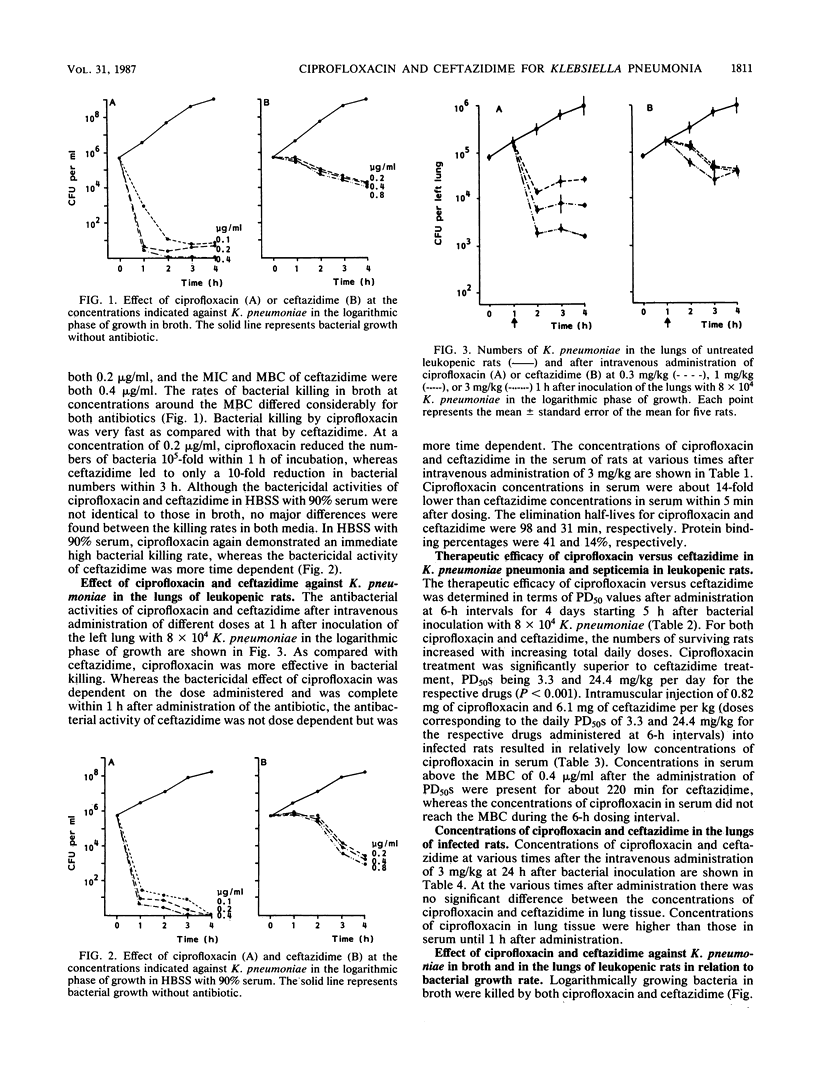
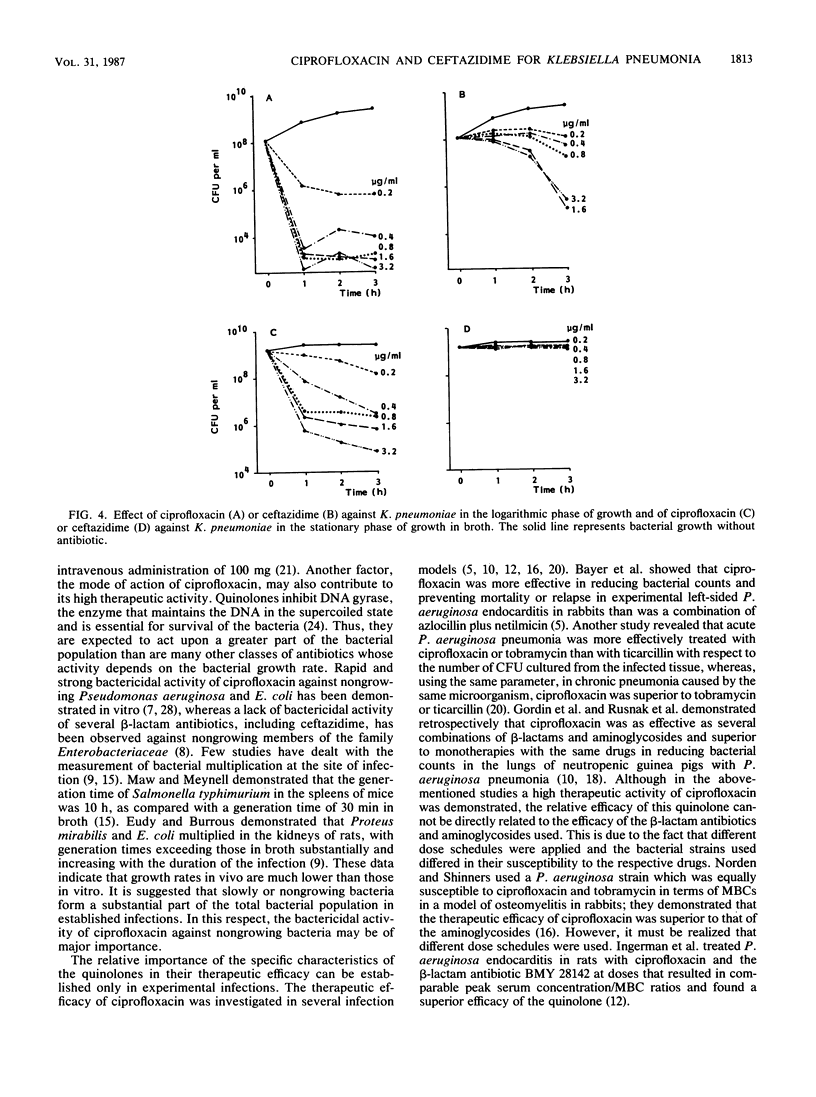
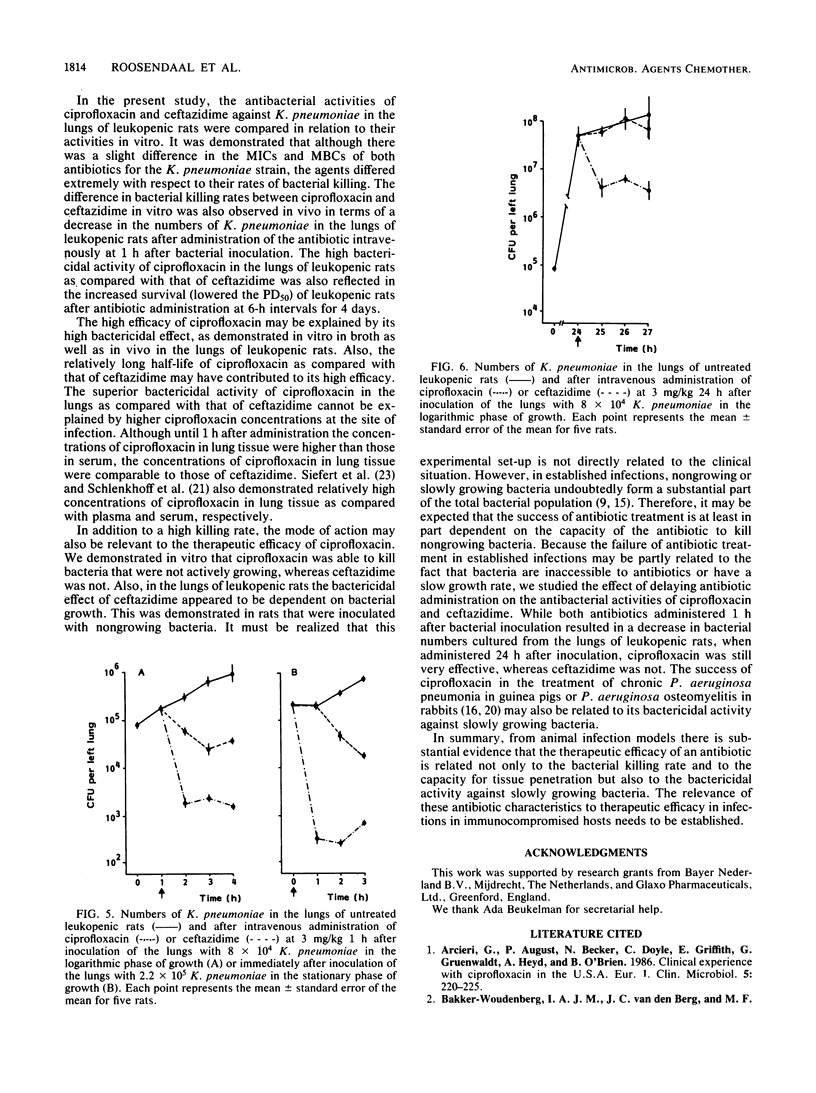
The antibacterial activities of ciprofloxacin and ceftazidime against Klebsiella pneumoniae in vitro and in vivo were compared. Although there was only a minor difference between the MBCs of both drugs, the bacterial killing rate of ciprofloxacin in vitro was very fast in comparison with that of ceftazidime. Similarly, the intravenous administration of ciprofloxacin at 1 h after bacterial inoculation resulted in effective bacterial killing in the lungs of leukopenic rats. This killing was dose dependent, in contrast to the dose-independent bactericidal effect of ceftazidime. The high antibacterial activity of ciprofloxacin in the lungs as compared with that of ceftazidime was also reflected in its therapeutic efficacy in K. pneumoniae pneumonia and septicemia in leukopenic rats when these infections were treated at 6-h intervals over 4 days, starting at 5 h after bacterial inoculation. Concentrations of ciprofloxacin and ceftazidime in lung tissue were not significantly different. Regarding the antibacterial activity of both drugs against K. pneumoniae in relation to the bacterial growth rate in vitro and in the lungs of leukopenic rats, ciprofloxacin killed K. pneumoniae organisms that were not actively growing, whereas ceftazidime did not. In addition, it was demonstrated that when the intravenous administration of antibiotic was delayed from 1 h up to 24 h after bacterial inoculation, ceftazidime lost its antibacterial activity in the lungs and blood of leukopenic rats, whereas ciprofloxacin was still very effective. These data suggest that the capacity of an antibiotic to kill bacteria at a slow growth rate may be relevant for its therapeutic effect in established infections, in which slowly growing bacteria form a substantial part of the total bacterial population.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arcieri G., August R., Becker N., Doyle C., Griffith E., Gruenwaldt G., Heyd A., O'Brien B. Clinical experience with ciprofloxacin in the USA. Eur J Clin Microbiol. 1986 Apr;5(2):220–225. doi: 10.1007/BF02013994. [DOI] [PubMed] [Google Scholar]
- Bakker-Woudenberg I. A., van den Berg J. C., Michel M. F. Therapeutic activities of cefazolin, cefotaxime, and ceftazidime against experimentally induced Klebsiella pneumoniae pneumonia in rats. Antimicrob Agents Chemother. 1982 Dec;22(6):1042–1050. doi: 10.1128/aac.22.6.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker-Woudenberg I. A., van den Berg J. C., Vree T. B., Baars A. M., Michel M. F. Relevance of serum protein binding of cefoxitin and cefazolin to their activities against Klebsiella pneumoniae pneumonia in rats. Antimicrob Agents Chemother. 1985 Nov;28(5):654–659. doi: 10.1128/aac.28.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball A. P. Overview of clinical experience with ciprofloxacin. Eur J Clin Microbiol. 1986 Apr;5(2):214–219. doi: 10.1007/BF02013993. [DOI] [PubMed] [Google Scholar]
- Bayer A. S., Blomquist I. K., Kim K. S. Ciprofloxacin in experimental aortic valve endocarditis due to Pseudomonas aeruginosa. J Antimicrob Chemother. 1986 May;17(5):641–649. doi: 10.1093/jac/17.5.641. [DOI] [PubMed] [Google Scholar]
- Bennett J. V., Brodie J. L., Benner E. J., Kirby W. M. Simplified, accurate method for antibiotic assay of clinical specimens. Appl Microbiol. 1966 Mar;14(2):170–177. doi: 10.1128/am.14.2.170-177.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalkley L. J., Koornhof H. J. Antimicrobial activity of ciprofloxacin against Pseudomonas aeruginosa, Escherichia coli, and Staphylococcus aureus determined by the killing curve method: antibiotic comparisons and synergistic interactions. Antimicrob Agents Chemother. 1985 Aug;28(2):331–342. doi: 10.1128/aac.28.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozens R. M., Tuomanen E., Tosch W., Zak O., Suter J., Tomasz A. Evaluation of the bactericidal activity of beta-lactam antibiotics on slowly growing bacteria cultured in the chemostat. Antimicrob Agents Chemother. 1986 May;29(5):797–802. doi: 10.1128/aac.29.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eudy W. W., Burrous S. E. Generation times of Proteus mirabilis and Escherichia coli in experimental infections. Chemotherapy. 1973;19(3):161–170. doi: 10.1159/000221451. [DOI] [PubMed] [Google Scholar]
- Gordin F. M., Hackbarth C. J., Scott K. G., Sande M. A. Activities of pefloxacin and ciprofloxacin in experimentally induced Pseudomonas pneumonia in neutropenic guinea pigs. Antimicrob Agents Chemother. 1985 Apr;27(4):452–454. doi: 10.1128/aac.27.4.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S. The fluoroquinolones: pharmacology, clinical uses, and toxicities in humans. Antimicrob Agents Chemother. 1985 Nov;28(5):716–721. doi: 10.1128/aac.28.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingerman M. J., Pitsakis P. G., Rosenberg A. F., Levison M. E. The importance of pharmacodynamics in determining the dosing interval in therapy for experimental pseudomonas endocarditis in the rat. J Infect Dis. 1986 Apr;153(4):707–714. doi: 10.1093/infdis/153.4.707. [DOI] [PubMed] [Google Scholar]
- Kramer B. S., Pizzo P. A., Robichaud K. J., Witesbsky F., Wesley R. Role of serial microbiologic surveillance and clinical evaluation in the management of cancer patients with fever and granulocytopenia. Am J Med. 1982 Apr;72(4):561–568. doi: 10.1016/0002-9343(82)90449-1. [DOI] [PubMed] [Google Scholar]
- Maw J., Meynell G. G. The true division and death rates of Salmonella typhimurium in the mouse spleen determined with superinfecting phage P22. Br J Exp Pathol. 1968 Dec;49(6):597–613. [PMC free article] [PubMed] [Google Scholar]
- Norden C. W., Shinners E. Ciprofloxacin as therapy for experimental osteomyelitis caused by Pseudomonas aeruginosa. J Infect Dis. 1985 Feb;151(2):291–294. doi: 10.1093/infdis/151.2.291. [DOI] [PubMed] [Google Scholar]
- Roosendaal R., Bakker-Woudenberg I. A., van den Berghe-van Raffe M., Michel M. F. Continuous versus intermittent administration of ceftazidime in experimental Klebsiella pneumoniae pneumonia in normal and leukopenic rats. Antimicrob Agents Chemother. 1986 Sep;30(3):403–408. doi: 10.1128/aac.30.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusnak M. G., Drake T. A., Hackbarth C. J., Sande M. A. Single versus combination antibiotic therapy for pneumonia due to Pseudomonas aeruginosa in neutropenic guinea pigs. J Infect Dis. 1984 Jun;149(6):980–985. doi: 10.1093/infdis/149.6.980. [DOI] [PubMed] [Google Scholar]
- Schiff J. B., Small G. J., Pennington J. E. Comparative activities of ciprofloxacin, ticarcillin, and tobramycin against experimental Pseudomonas aeruginosa pneumonia. Antimicrob Agents Chemother. 1984 Jul;26(1):1–4. doi: 10.1128/aac.26.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenkhoff D., Dalhoff A., Knopf J., Opferkuch W. Penetration of ciprofloxacin into human lung tissue following intravenous injection. Infection. 1986 Nov-Dec;14(6):299–300. doi: 10.1007/BF01643967. [DOI] [PubMed] [Google Scholar]
- Sculier J. P., Weerts D., Klastersky J. Causes of death in febrile granulocytopenic cancer patients receiving empiric antibiotic therapy. Eur J Cancer Clin Oncol. 1984 Jan;20(1):55–60. doi: 10.1016/0277-5379(84)90034-8. [DOI] [PubMed] [Google Scholar]
- Siefert H. M., Maruhn D., Scholl H. Pharmacokinetics of ciprofloxacin. 2nd communication: distribution to and elimination from tissues and organs following single or repeated administration of [14C]ciprofloxacin in albino rats. Arzneimittelforschung. 1986 Oct;36(10):1503–1510. [PubMed] [Google Scholar]
- Smith J. T. Wirkmechanismus der Chinolone. Infection. 1986;14 (Suppl 1):S3–15. doi: 10.1007/BF01645191. [DOI] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Edwards L. J. In vitro activity of Bay 09867, a new quinoline derivative, compared with those of other antimicrobial agents. Antimicrob Agents Chemother. 1983 Apr;23(4):559–564. doi: 10.1128/aac.23.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Lockley R. M., Webberly M., Dent J. Pharmacokinetics of intravenously administered ciprofloxacin. Antimicrob Agents Chemother. 1984 Aug;26(2):208–210. doi: 10.1128/aac.26.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrob Agents Chemother. 1985 Oct;28(4):581–586. doi: 10.1128/aac.28.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiler H. J. Evaluation of the in vitro bactericidal action of ciprofloxacin on cells of Escherichia coli in the logarithmic and stationary phases of growth. Antimicrob Agents Chemother. 1985 Oct;28(4):524–527. doi: 10.1128/aac.28.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]


