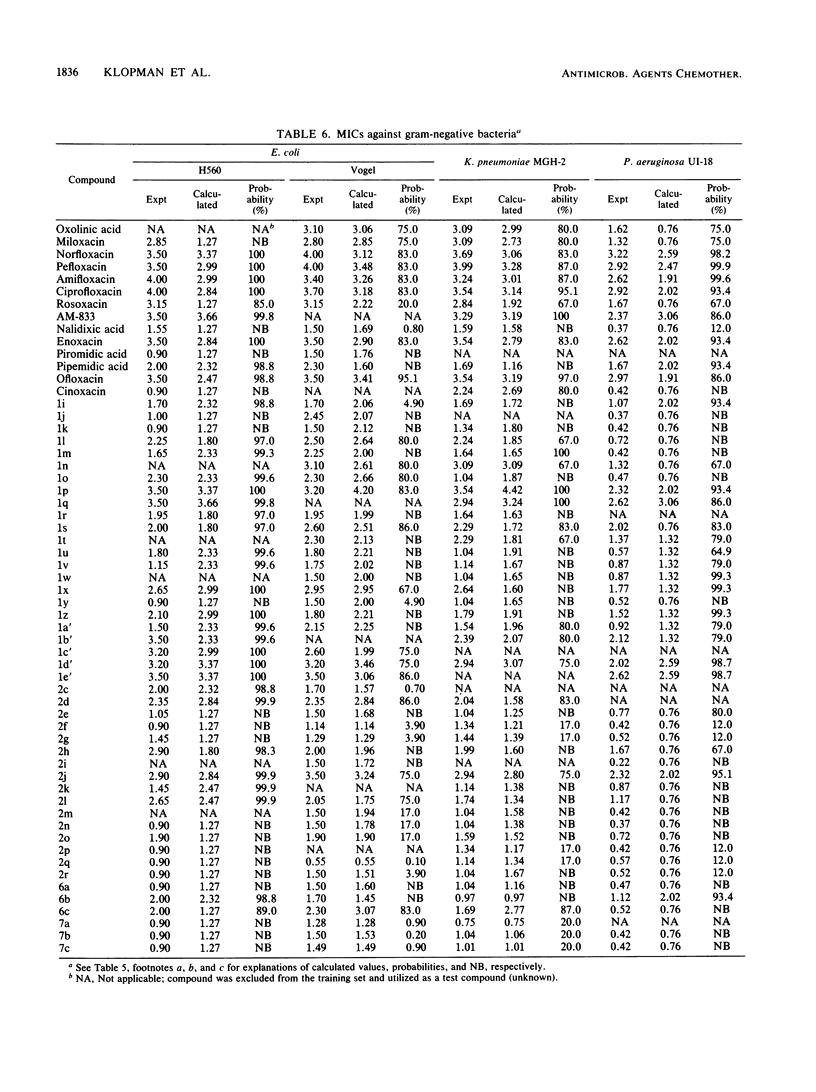
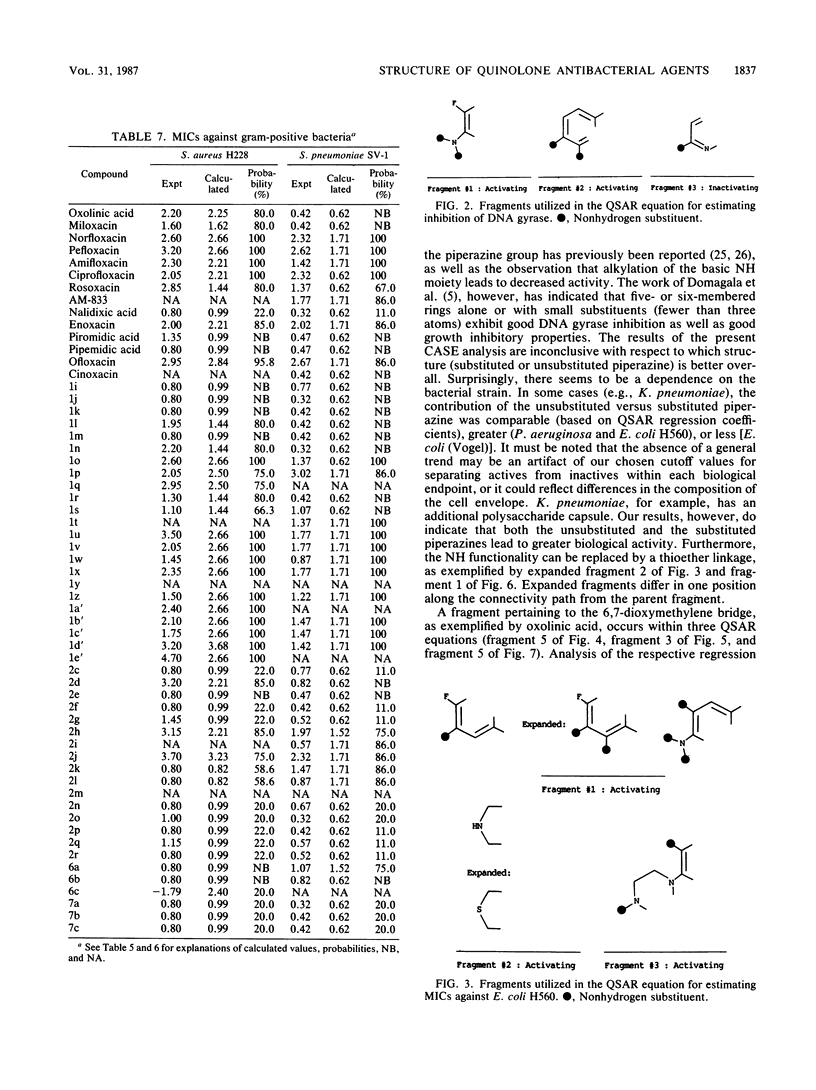
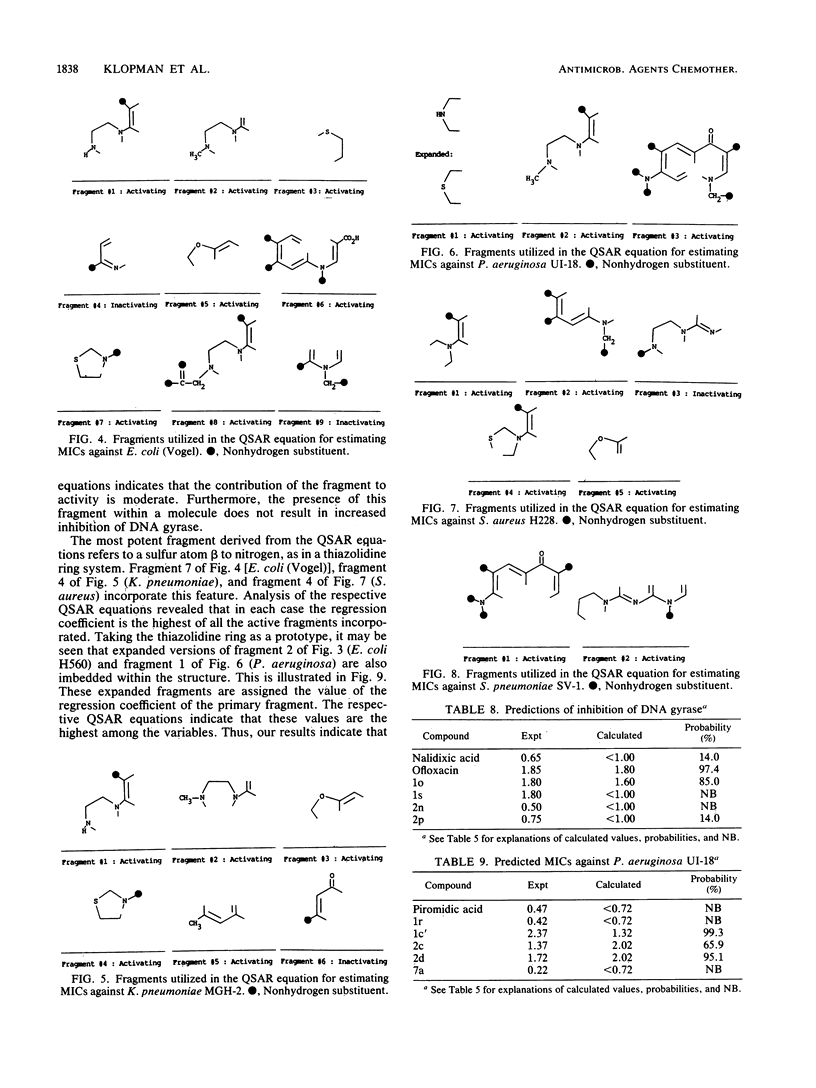
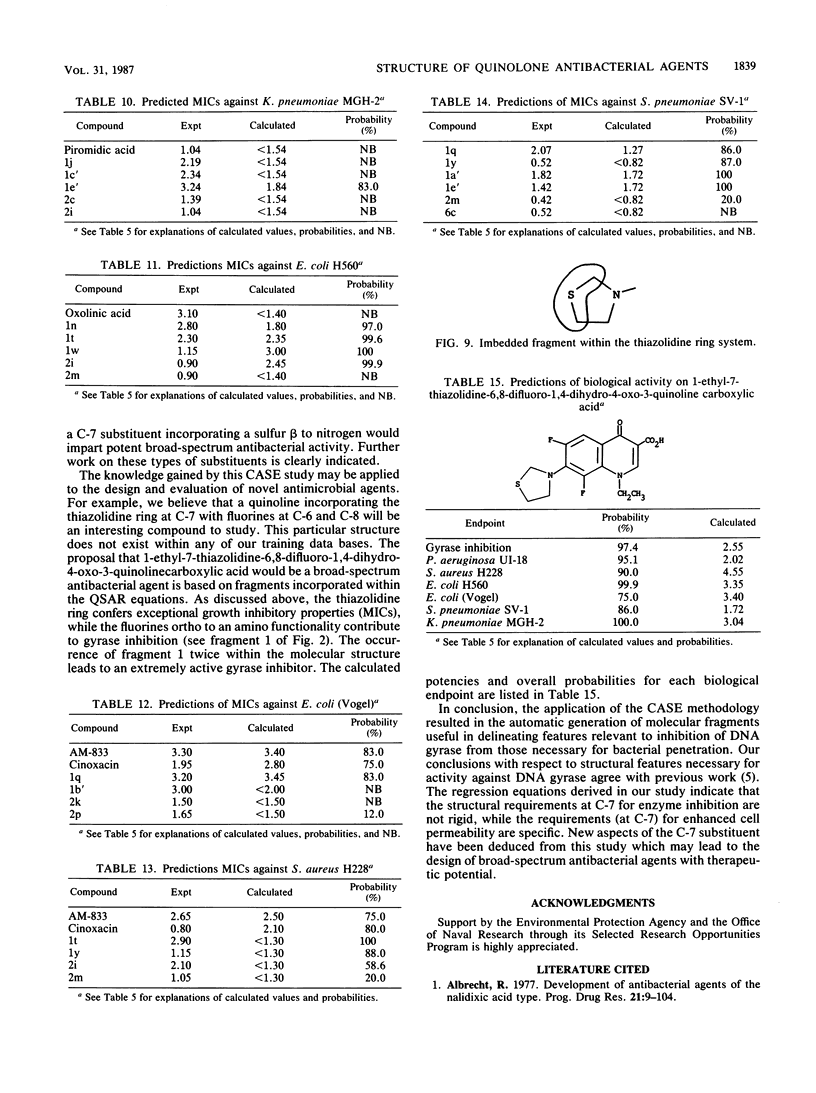
Abstract
The Computer Automated Structure Evaluation (CASE) program was used to study a series of quinolone antibacterial agents for which experimental data pertaining to DNA gyrase inhibition as well as MICs against several strains of gram-positive and gram-negative bacteria are available. The result of the analysis was the automatic generation of molecular fragments relevant to the respective biological endpoints. The potential significance of these major activating-inactivating fragments to the biological activity is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht R. Development of antibacterial agents of the nalidixic acid type. Prog Drug Res. 1977;21:9–104. doi: 10.1007/978-3-0348-7098-6_1. [DOI] [PubMed] [Google Scholar]
- Benbrook D. M., Miller R. V. Effects of norfloxacin on DNA metabolism in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1986 Jan;29(1):1–6. doi: 10.1128/aac.29.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D. T., Fernandes P. B., Claiborne A. K., Pihuleac E., Nordeen C. W., Maleczka R. E., Jr, Pernet A. G. Synthesis and structure-activity relationships of novel arylfluoroquinolone antibacterial agents. J Med Chem. 1985 Nov;28(11):1558–1564. doi: 10.1021/jm00149a003. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R. DNA gyrase and the supercoiling of DNA. Science. 1980 Feb 29;207(4434):953–960. doi: 10.1126/science.6243420. [DOI] [PubMed] [Google Scholar]
- Domagala J. M., Hanna L. D., Heifetz C. L., Hutt M. P., Mich T. F., Sanchez J. P., Solomon M. New structure-activity relationships of the quinolone antibacterials using the target enzyme. The development and application of a DNA gyrase assay. J Med Chem. 1986 Mar;29(3):394–404. doi: 10.1021/jm00153a015. [DOI] [PubMed] [Google Scholar]
- Domagala J. M., Heifetz C. L., Mich T. F., Nichols J. B. 1-Ethyl-7-[3-[(ethylamino)methyl]-1-pyrrolidinyl]-6,8-difluoro-1,4- dihydro-4-oxo-3-quinoline-carboxylic acid. New quinolone antibacterial with potent gram-positive activity. J Med Chem. 1986 Apr;29(4):445–448. doi: 10.1021/jm00154a003. [DOI] [PubMed] [Google Scholar]
- Egawa H., Miyamoto T., Minamida A., Nishimura Y., Okada H., Uno H., Matsumoto J. Pyridonecarboxylic acids as antibacterial agents. 4. Synthesis and antibacterial activity of 7-(3-amino-1-pyrrolidinyl)-1-ethyl-6-fluoro-1,4 -dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acid and its analogues. J Med Chem. 1984 Dec;27(12):1543–1548. doi: 10.1021/jm00378a004. [DOI] [PubMed] [Google Scholar]
- FREE S. M., Jr, WILSON J. W. A MATHEMATICAL CONTRIBUTION TO STRUCTURE-ACTIVITY STUDIES. J Med Chem. 1964 Jul;7:395–399. doi: 10.1021/jm00334a001. [DOI] [PubMed] [Google Scholar]
- Frierson M. R., Klopman G., Rosenkranz H. S. Structure-activity relationships (SARs) among mutagens and carcinogens: a review. Environ Mutagen. 1986;8(2):283–327. doi: 10.1002/em.2860080210. [DOI] [PubMed] [Google Scholar]
- GOSS W. A., DEITZ W. H., COOK T. M. MECHANISM OF ACTION OF NALIDIXIC ACID ON ESCHERICHIA COLI.II. INHIBITION OF DEOXYRIBONUCLEIC ACID SYNTHESIS. J Bacteriol. 1965 Apr;89:1068–1074. doi: 10.1128/jb.89.4.1068-1074.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Itoh T., Tomizawa J. I. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4772–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Irikura T., Iyobe S., Mitsuhashi S. Differences in susceptibility to quinolones of outer membrane mutants of Salmonella typhimurium and Escherichia coli. Antimicrob Agents Chemother. 1986 Mar;29(3):535–538. doi: 10.1128/aac.29.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Suzue S., Irikura T., Iyobe S., Mitsuhashi S. Isolation and characterization of norfloxacin-resistant mutants of Escherichia coli K-12. Antimicrob Agents Chemother. 1986 Aug;30(2):248–253. doi: 10.1128/aac.30.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Souza K. S., Tung C., McHugh G. L., Swartz M. N. Genetic and biochemical characterization of norfloxacin resistance in Escherichia coli. Antimicrob Agents Chemother. 1986 Apr;29(4):639–644. doi: 10.1128/aac.29.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Högberg T., Khanna I., Drake S. D., Mitscher L. A., Shen L. L. Structure-activity relationships among DNA gyrase inhibitors. Synthesis and biological evaluation of 1,2-dihydro-4, 4-dimethyl-1-oxo-2-naphthalenecarboxylic acids as 1-carba bioisosteres of oxolinic acid. J Med Chem. 1984 Mar;27(3):306–310. doi: 10.1021/jm00369a013. [DOI] [PubMed] [Google Scholar]
- Klopman G., Contreras R. Use of artificial intelligence in structure-activity correlations of anticonvulsant drugs. Mol Pharmacol. 1985 Jan;27(1):86–93. [PubMed] [Google Scholar]
- Klopman G., Frierson M. R., Rosenkranz H. S. Computer analysis of toxicological data bases: mutagenicity of aromatic amines in Salmonella tester strains. Environ Mutagen. 1985;7(5):625–644. doi: 10.1002/em.2860070503. [DOI] [PubMed] [Google Scholar]
- Klopman G., Macina O. T. Use of the Computer Automated Structure Evaluation program in determining quantitative structure-activity relationships within hallucinogenic phenylalkylamines. J Theor Biol. 1985 Apr 21;113(4):637–648. doi: 10.1016/s0022-5193(85)80184-3. [DOI] [PubMed] [Google Scholar]
- Koga H., Itoh A., Murayama S., Suzue S., Irikura T. Structure-activity relationships of antibacterial 6,7- and 7,8-disubstituted 1-alkyl-1,4-dihydro-4-oxoquinoline-3-carboxylic acids. J Med Chem. 1980 Dec;23(12):1358–1363. doi: 10.1021/jm00186a014. [DOI] [PubMed] [Google Scholar]
- Matsumoto J., Miyamoto T., Minamida A., Nishimura Y., Egawa H., Nishimura H. Pyridonecarboxylic acids as antibacterial agents. 2. Synthesis and structure-activity relationships of 1,6,7-trisubstituted 1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acids, including enoxacin, a new antibacterial agent. J Med Chem. 1984 Mar;27(3):292–301. doi: 10.1021/jm00369a011. [DOI] [PubMed] [Google Scholar]
- McCoy E. C., Petrullo L. A., Rosenkranz H. S. Non-mutagenic genotoxicants: novobiocin and nalidixic acid, 2 inhibitors of DNA gyrase. Mutat Res. 1980 Sep;79(1):33–43. doi: 10.1016/0165-1218(80)90145-7. [DOI] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topliss J. G., Edwards R. P. Chance factors in studies of quantitative structure-activity relationships. J Med Chem. 1979 Oct;22(10):1238–1244. doi: 10.1021/jm00196a017. [DOI] [PubMed] [Google Scholar]
- Winshell E. B., Rosenkranz H. S. Nalidixic Acid and the Metabolism of Escherichia coli. J Bacteriol. 1970 Dec;104(3):1168–1175. doi: 10.1128/jb.104.3.1168-1175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi J., Furutani Y., Inoue S., Ohue T., Nakamura S., Shimizu M. New nalidixic acid resistance mutations related to deoxyribonucleic acid gyrase activity. J Bacteriol. 1981 Nov;148(2):450–458. doi: 10.1128/jb.148.2.450-458.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweerink M. M., Edison A. Inhibition of Micrococcus luteus DNA gyrase by norfloxacin and 10 other quinolone carboxylic acids. Antimicrob Agents Chemother. 1986 Apr;29(4):598–601. doi: 10.1128/aac.29.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]



