Abstract
Neuronal NMDA receptors (NMDARs) colocalize with postsynaptic density protein-95 (PSD-95), a putative NMDAR anchoring protein and core component of the PSD, at excitatory synapses. PKC activation and PSD-95 expression each enhance NMDAR channel opening rate and number of functional channels at the cell surface. Here we show in Xenopus oocytes that PSD-95 and PKC potentiate NMDA gating and trafficking in a nonadditive manner. PSD-95 and PKC each enhance NMDA channel activity, with no change in single-channel conductance, reversal potential or mean open time. PSD-95 and PKC each potentiate NMDA channel opening rate (kβ) and number of functional channels at the cell surface (N), as indicated by more rapid current decay and enhanced charge transfer in the presence of the open channel blocker MK-801. PSD-95 and PKC each increase NMDAR surface expression, as indicated by immunofluorescence. PKC potentiates NMDA channel function and NMDAR surface expression to the same final absolute values in the absence or presence of PSD-95. Thus, PSD-95 partially occludes PKC potentiation. We further show that Ser-1462, a putative phosphorylation target within the PDZ-binding motif of the NR2A subunit, is required for PSD-95-induced potentiation and partial occlusion of PKC potentiation. Coimmunoprecipitation experiments with cortical neurons in culture indicate that PKC activation promotes assembly of NR2 with NR1, and that the newly assembled NMDARs are not associated with PSD-95. These findings predict that synaptic scaffolding proteins and protein kinases convergently modulate NMDAR gating and trafficking at synaptic sites.
Keywords: channel gating, PDZ proteins, phosphorylation
NMDA receptors (NMDARs) are glutamate-gated ion channels and are localized to excitatory synapses throughout the brain. Each receptor molecule is a tetramer of NR1 and NR2A-D subunits, which assemble in the endoplasmic reticulum to form functional channels with differing channel properties (1). Further molecular diversity arises by alternative splicing of the NR1 subunit (2). Targeting of NMDARs to synaptic sites is dynamically regulated in an activity-dependent manner and is thought to play a role in normal synaptic transmission and in some forms of NMDAR-dependent synaptic plasticity (3–5). Assembled NMDARs are targeted selectively to the postsynaptic side of glutamatergic synapses (6) and appear (together with AMPA receptors) at nascent synapses within 1–2 h of initial axodendritic contact (7). NMDARs are primarily NR2B-containing at early ages; as neurons mature, there is a progressive inclusion of NR2A subunits in synaptic NMDARs (8).
Synaptic NMDARs are localized to postsynaptic densities (PSDs) at excitatory synapses, where they are structurally organized (and spatially restricted) in a large macromolecular signaling complex composed of scaffolding and adaptor proteins, which physically link the receptors to kinases, phosphoprotein phosphatases, and other signaling molecules (9–12). Synaptic scaffolding proteins not only regulate receptor trafficking and anchoring at synaptic sites but also regulate receptor function. Overexpression of PSD-95 promotes NMDAR clustering (13), accelerates maturation of excitatory synapses (14), reduces NMDAR desensitization (15), and, at cerebellar granule cell synapses, preferentially stabilizes NR1/NR2A (vs. NR1/NR2B) receptors (16). PSD-95 expression in heterologous cells increases the NMDA channel opening rate (17), enhances receptor insertion (17), and inhibits receptor internalization (17–19), thereby enhancing the residence time of receptors at the cell surface.
Regulation of neuronal NMDARs by protein kinases plays a critical role in synaptic transmission and synaptic plasticity of NMDAR excitatory postsynaptic currents (EPSCs; refs. 20 and 21). PKC potentiates NMDA currents and single channel activity in recombinant expression systems and in neurons (22, 23). PKC activation promotes trafficking of new channels to the cell surface by SNARE-dependent exocytosis and enhances the NMDA channel-opening rate (23), mechanisms implicated in long-term potentiation of NMDAR–EPSCs at mature hippocampal CA1 synapses (24). Activity- and casein kinase II-dependent phosphorylation of Ser-1480 within the PDZ-binding motif of the NMDAR NR2B subunit disrupts the interaction of NMDARs with PSD-95 and SAP102 and reduces NMDAR surface expression at synaptic sites (25). These findings demonstrate that kinase-dependent phosphorylation regulates interaction of synaptic scaffolding proteins with NMDARs. Conversely, PSD-95 attenuates PKC potentiation of NMDARs expressed as the ratio of potentiated to control responses (26–28). However, the molecular mechanisms by which synaptic scaffolding proteins modulate kinase effects on NMDAR gating and trafficking are not well delineated.
The present study demonstrates that association of PSD-95 with synaptic NMDARs partially occludes PKC potentiation in Xenopus oocytes. Xenopus oocytes provide geometric simplicity and enable analysis of a homogenous population of receptors. Experiments involving whole-cell and patch–clamp recording and cell-surface immunolabeling reveal that PSD-95 increases NMDAR surface expression and the opening rate of NR2A receptors, but that final values after PKC potentiation are unaffected by PSD-95 expression. Thus, PSD-95 reduces the PKC potentiation ratio for these parameters. Site-directed mutagenesis reveals that Ser-1480 within the ESDV (PDZ-binding) motif of the NR2A subunit is required for the effects of PSD-95 on NMDARs. Coimmunoprecipitation of neuronal proteins reveals that PKC activation promotes assembly of NMDARs but does not alter the number of NMDARs associated with PSD-95. PSD-95/SAP90 family proteins appear strategically poised to modify kinase-dependent regulation of NMDAR gating and trafficking at synaptic sites.
Results
PSD-95 and PKC Potentiate NMDA-Elicited Currents in a Nonadditive Manner.
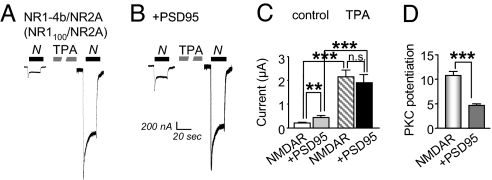
We examined the impact of PSD-95 expression on PKC potentiation of NMDAR gating and trafficking in Xenopus oocytes expressing NR1–4b (NR1100)/NR2A receptors. In a subset of cells, PSD-95 mRNA was injected 48 h after injection of NMDAR mRNAs, and responses were elicited 24 h later. NR1–4b/NR2A receptors exhibit the greatest PKC potentiation ratios (22). Currents were elicited by bath application of NMDA (100 μM) with glycine (10 μM). Coexpression of PSD-95 with NMDARs enhanced basal NMDA-elicited currents (potentiation to ≈2-fold of control; Fig. 1A–C). The effect of PSD-95 on basal NMDA currents increased with increasing quantity of PSD-95 mRNA injected and was maximal at 20 ng of mRNA per cell, the amount used in these experiments (data not illustrated). Activation of PKC by application of 12-O-tetradecanoyl phorbol-13-acetate (TPA) potentiated NMDA currents to nearly the same final value in the absence and presence of PSD-95, and the potentiation ratios were ≈10- and ≈5-fold, respectively (Fig. 1C). Thus, PSD-95 expression enhanced basal NMDA currents and partially occluded PKC potentiation (Fig. 1D).
Fig. 1.
PSD-95 expression partially occludes PKC potentiation of NMDA-elicited currents. NMDA-elicited currents (100 μM NMDA with 10 μM glycine) were recorded in Ca2+ Ringer's solution at a holding potential of −60 mV from oocytes expressing NR1–4b/NR2A receptors in the absence or presence of PSD-95. (A and B) PKC activated by TPA potentiated NMDA-elicited currents in oocytes expressing NR1/NR2A receptors (Left). Expression of PSD-95 increased the initial or basal NMDA-elicited currents by ≈2-fold, but PKC potentiated NMDA currents to similar final values in the presence and absence of PSD-95 (Right). (C) Quantitation of these data. (D) Because PSD-95 increased the basal response but not the response after TPA, the potentiation ratio was reduced from ≈10- to ≈5-fold. ∗∗, P < 0.01; ∗∗∗, P < 0.001. n.s., not significant.
PSD-95 and PKC Potentiate NMDA Channel Activity in a Nonadditive Manner.
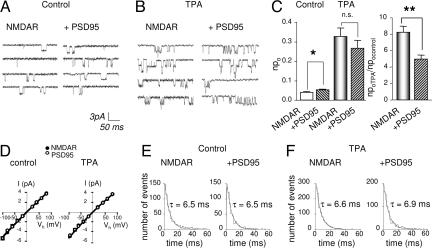
To examine mechanistically the interactions between PSD-95 and PKC potentiation, we recorded NMDA channel activity in outside-out patches excised from oocytes expressing NMDARs in the absence or presence of PSD-95. In patches excised from cells expressing NMDARs alone or with PSD-95, NMDA (10 μM plus glycine) activated channels with a conductance, γ = 50 ± 1 pS, which did not vary with voltage; there was no change in reversal potential, Erev = ≈0 mV (n = 5; Fig. 2A–D). PSD-95 increased the number of active channels times channel open probability, npo, to ≈1.3-fold of control (NMDAR cells, 0.040 ± 0.004, n = 5; NMDAR + PSD-95 cells, 0.052 ± 0.003, n = 5, P < 0.01; Fig. 2 A–C). PKC activation potentiated npo by ≈8-fold in the absence of PSD-95 and ≈5-fold in the presence of PSD-95 to nearly the same final values (NMDAR cells, 0.33 ± 0.04, n = 5; P < 0.001 for TPA vs. control; NMDAR + PSD-95 cells, 0.27 ± 0.04, n = 5; P < 0.001 for TPA vs. control; Fig. 2 A–C). Thus, PSD-95 potentiation of npo partially occluded PKC potentiation (Fig. 2C Right). Neither PSD-95 expression, PKC activation, nor both together detectably altered single channel conductance or reversal potential (Fig. 2D) or mean open time (τ = ≈6.5 for all four groups; n = 5 per group; not significant for all pairwise comparisons; Fig. 2 E and F).
Fig. 2.
PSD-95 partially occludes PKC potentiation of NMDA single channel activity. Representative traces of NMDA single channel activity recorded from outside-out patches excised from oocytes expressing NR1–4b/NR2A receptors in the absence or presence of PSD-95. (A–C) PDS-95 expression enhanced NMDA channel number times open probability, npo by ≈1.3-fold. (B and C) PKC activation by TPA markedly increased npo to nearly the same final value in cells expressing NMDARs in the absence or presence of PSD-95. (D) Neither PSD-95 nor PKC activation, nor the two together, detectably altered single channel conductance; the slope of single channel currents was linear from −100 to + 80 mV in all four conditions (white circles denoting NMDARs + PSD-95 obscure underlying black circles for NMDARs alone). NMDA activated single channels with a unitary conductance (γ = 50 ± 1 pS; Erev ≈ 0 mV) that was the same in the absence or presence of PSD-95 before and after PKC activation; n = 5 per group). (E and F) Mean open time distributions were the same in all four conditions. Responses were elicited by application of NMDA (10 μM with 10 μM glycine) at a holding potential of −60 mV. To activate PKC, TPA (100 nM) was bath-applied. ∗, P < 0.05; ∗∗, P < 0.01.
PSD-95 and PKC Increase Channel Number and Opening Rate in a Nonadditive Manner.
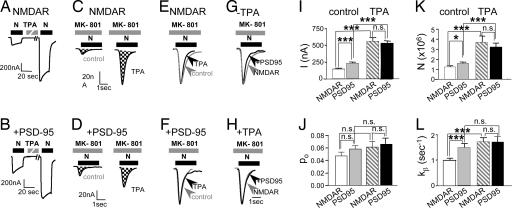
The results reported thus far indicate that PSD-95 expression and PKC activation each increases channel open probability and/or the number of active NMDA channels at the cell surface. To analyze independently the effects of PSD-95 and of PKC on the number of functional channels, N, and open probability, po, we used the essentially irreversible NMDA open-channel blocker MK-801 (17, 23). We recorded NMDA-elicited currents from cells expressing NR1–4b/NR2A receptors in the absence (Fig. 3A) or presence (Fig. 3B) of PSD-95. PSD-95 expression increased NMDA currents to ≈1.6-fold of control, and the final current after PKC potentiation was the same in the presence and absence of PSD-95 (Fig. 3 A, B, and I).
Fig. 3.
PSD-95 partially occludes PKC potentiation of NMDA channel number and opening rate. (A and B) Sample records of NMDA-elicited currents recorded from oocytes expressing NR1–4b/NR2A receptors in the absence or presence of PSD-95 and before and after TPA application in Ca2+- free (Ba2+) Ringer's. (C and D) Responses in the absence and presence of PSD-95 in the continuous presence of the open channel blocker MK-801 applied shortly before NMDA (5 μM) to control (Left) and TPA-treated (Right) oocytes. Charge transfer is indicated by cross-hatching. Different oocytes were used for each condition. Coexpression of PSD-95 increased the initial (basal) NMDA-elicited current (compare A with B) and the number of functional NMDA channels, N, at the cell surface under basal conditions (compare C with D). TPA increased N to the same final value in cells expressing NMDARs alone vs. NMDARs with PSD-95, but the potentiation ratio was smaller in cells coexpressing PSD-95 (compare C with D). (E–H), Responses in C and D were normalized to the same peak amplitude for pairwise comparison of the time course of response decay in MK-801, a measure of opening rate, kβ. (E) TPA increased the rate of decay of NMDA currents recorded from cells expressing NMDARs in the absence of PSD-95, indicating an increase in the channel opening rate, kβ. (F) TPA did not detectably alter kβ in cells coexpressing PSD-95. (G) The rate of decay of NMDA currents was greater in the presence than the absence of PSD-95. (H) After TPA treatment, the rate of decay of NMDA currents did not differ in the absence and presence of PSD-95. Increase in kβ caused by coexpression of PSD-95 occludes PKC potentiation of kβ. (I–L) Summary of data in experiments illustrated in A–H. In the presence of PSD-95, I, N, and kβ, but not po, were greater than in its absence, but the final values after TPA potentiation were not significantly different. Thus, PSD-95 expression occludes the TPA potentiation ratios. Currents were elicited by application of NMDA (1 mM NMDA with 50 μM glycine) at a holding potential of −60 mV.
For oocytes expressing NMDARs in the absence of PSD-95, the mean number of channels per cell, Ncontrol [calculated from cumulative charge transfer, Q (17, 23)] was 1.24 ± 0.23 × 106 (n = 21). Because PSD-95 expression does not detectably alter channel conductance or reversal potential (Fig. 2 A, B, and D) and does not change the affinity of NMDARs for MK-801 at high agonist concentration (29), PSD-95 should not affect kbl, the rate constant of block. From the measured increase in Q, PSD-95 expression increased N to ≈1.3-fold of control (P < 0.05; Fig. 3 C, D, and J), a value that accounts for a major component of the increase in NMDA current measured at the peak of the response before MK-801 application (IPSD/Icontrol = 1.6, n = 28 and 26, respectively; P < 0.001; Fig. 3I). PKC activation increased N to 3.7-fold of control in the absence of PSD-95 (NTPA/Ncontrol = 2.99; n = 16 and 21, respectively; Fig. 3 C, D, and K), a value that accounts for a major component of the PKC potentiation of NMDA-elicited current (ITPA/Icontrol = 3.88, n = 19 and 26, respectively; Fig. 3I). PKC activation potentiated N to 3.2-fold of control in the presence of PSD-95, to nearly the same final value as in the absence of PSD-95 (NMDAR cells, 3.7 ± 0.6 × 106; n = 16; NMDAR + PSD-95 cells, 3.2 ± 0.4 × 106; n = 13, not significant; Fig. 3 C, D, and K). Thus, PSD-95 reduced the degree of PKC potentiation, measured as the ratio of the number of functional channels per cell after and before PKC activation (NTPA/Ncontrol = 2.99 in the absence of PSD-95, NTPA/Ncontrol = 2.00 in the presence of PSD-95, n = 16 and 13; Fig. 3K). Neither PSD-95 expression nor PKC activation, nor the two together, significantly altered channel open probability, po [calculated from I and N (17, 23); Fig. 3J]. Thus, the major effect of PSD-95 and PKC is to increase the number of functional channels at the cell surface, and the PSD-95-induced increase partially occludes PKC potentiation. These findings confirm and extend our earlier studies of PKC potentiation and PSD-95 expression evaluated separately (17, 23).
As an independent measure of the effect of PSD-95 and PKC on NMDA channel gating, we analyzed the rate of decay of NMDA-elicited currents in the presence of MK-801 (Fig. 3 E–H). Provided that kbl[MK-801] ≫ kα and kβ (the closing and opening rates, respectively), the late decay of the current can be described by a single exponential with a rate constant equal to the opening rate constant, kβ. Association with PSD-95 increased kβ to ≈1.5-fold of control (NMDAR cells, 1.00 ± 0.05 s−1, n = 14; NMDAR + PSD-95 cells, 1.51 ± 0.10 s−1; n = 20; P < 0.001; Fig. 3L). PKC increased the channel opening rate kβ to nearly the same final value in the absence or presence of PSD-95 (NMDAR cells, 1.74 ± 0.16 s−1, n = 17; NMDAR + PSD-95 cells, 1.75 ± 0.18 s−1; n = 15; n.s. vs. NMDAR + PSD-95 cells; Fig. 3L). In this case, PSD-95 expression completely occluded the effect of PKC potentiation in increasing kβ.
In contrast, neither PSD-95 nor PKC significantly altered channel closing rate, kα (NMDAR cells, 20 ± 2 s−1; NMDAR + PSD-95 cells, 27 ± 5 s−1; NMDAR cells after TPA, kα 25 ± 3 s−1; NMDAR + PSD-95 cells after TPA, kα 25 ± 4 s−1; n = 14–20 per group; not significant for all pairwise comparisons; data not illustrated). A summary of the effects of PSD-95 expression and PKC potentiation on NMDARs is presented in supporting information (SI) Table 1.
PSD-95 and PKC Enhance NMDAR Surface Expression in a Nonadditive Manner.
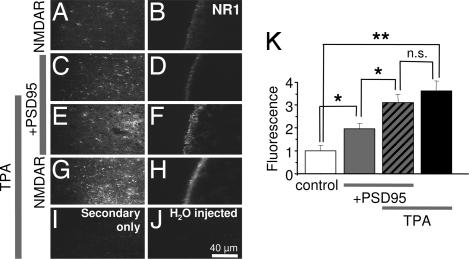
PKC activation and PSD-95 expression enhance NMDAR surface expression assessed by immunofluorescence with an antibody that recognizes the N-terminal domain of the NR1 subunit (17, 23). We measured immunofluorescence of intact, nonpermeabilized cells expressing NMDARs in the absence or presence of PSD-95. Control cells expressing NMDARs alone exhibited clear immunofluorescence that appeared confined to the external surface in cross-sectional and tangential images (Fig. 4A and B). PSD-95 expression enhanced NR1 surface fluorescence to ≈2-fold of control (Fig. 4 C, D, and K). PKC increased NR1 surface fluorescence to ≈3.6 ± 0.4-fold of control in the absence (n = 12; P < 0.01) and ≈1.6 ± 0.2-fold in the presence (n = 10; P < 0.05; Fig. 4 G, H, and K) of PSD-95. As observed for channel number, the fluorescence level after PKC activation was the same with or without PSD-95; thus, PSD-95 potentiation of NMDAR surface expression partially occluded PKC potentiation. Little or no immunofluorescence was observed for cells reacted with FITC-tagged secondary antibody in the absence of primary antibody (Fig. 4I) or for control cells (H2O-injected) labeled with primary and secondary antibodies (Fig. 4J). These findings verify the specificity of the immunofluorescence labeling.
Fig. 4.
PSD-95 partially occludes PKC potentiation of surface NMDAR expression. Representative tangential (left column) and cross-sectional (right column) images of oocytes expressing NR1–4b/NR2A receptors in the absence (A and B) or presence (C and D) of PSD-95. (E–H) TPA increased NMDAR surface expression to nearly the same final value in the absence or presence of PSD-95. (I and J) Oocytes expressing NMDARs labeled with secondary antibody in the absence of primary antibody (I) or water-injected oocytes labeled as in A--H (J) showed negligible fluorescence. (K) Summary of data in A--J. ∗, P < 0.05; ∗∗, P < 0.01.
Nonadditivity of PSD-95 and PKC Is Independent of NR1 Alternative Splicing.
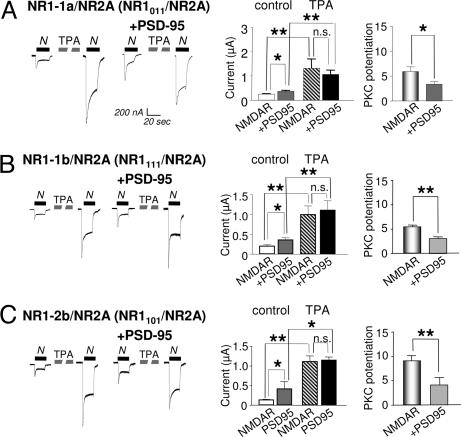
Alternative splicing of the NR1 C-terminal tail markedly affects the degree of PKC potentiation of NMDARs (22). Whereas NR1–4b/NR2A receptors exhibit ≈10-fold potentiation (Fig. 1D), and NR1–1a/NR2A receptors exhibit ≈6 fold potentiation (Fig. 5A). In cells expressing NR1–1a/NR2A receptors, PSD-95 increased NMDA-elicited currents ≈1.3-fold (Fig. 5A Left). For each splice variant, PKC potentiated the NMDA current to nearly the same final value in the absence or presence of PSD-95, consistent with partial occlusion of PKC potentiation by PSD-95. In the case of NR1–1a/NR2A receptors, PSD-95 reduced PKC the potentiation ratio from ≈6- to ≈3-fold (P < 0.05; Fig. 5A Right). Similar results were observed for NR1–1b/NR2A receptors, indicating that the N1 splice cassette had little or no effect on potentiation by PSD-95 or PKC alone or in combination (Fig. 5B). For NR1–2b/NR2A receptors, again, the final NMDA current after PKC potentiation was the same in the absence or presence of PSD-95, and PSD-95 reduced PKC potentiation from ≈9- to ≈4 fold (Fig. 5C). Similar results were obtained for NR1–4b/NR2A receptors (Fig. 1), indicating that exchange of C2′ cassette for the C2 cassette had little or no effect on potentiation by PSD-95 or PKC alone or in combination. The C2′ cassette contains a putative PDZ-binding motif, which appears to play little or no role in the regulation of NMDAR trafficking or gating by either PSD-95 or PKC. Moreover, truncation of the NR1 subunit, which removes the C1, C2, and C2′ cassettes, was without effect (not illustrated).
Fig. 5.
PSD-95-induced occlusion of PKC potentiation is not regulated by NR1 splicing. (A–C) NMDA-elicited currents recorded from oocytes expressing NR1–1a/NR2A (A), NR1–1b/NR2A (B), or NR1–2b/NR2A (C) receptors alone or together with PSD-95 before and after PKC activation. Responses were elicited by NMDA (300 μM) with glycine (10 μM) and recorded in Ca2+ Ringer's solution at a holding potential of −60 mV. For each splice variant, PKC potentiated NMDA-elicited currents to nearly the same final responses in the absence or presence of PSD-95. Thus, the PKC potentiation ratio was reduced in cells expressing PSD-95.
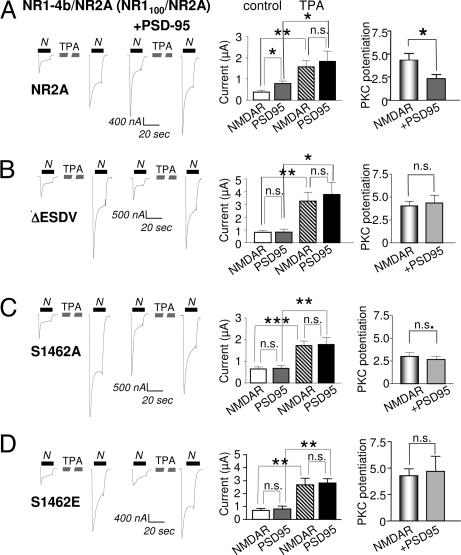
Ser-1462 in NR2A Is Required for PSD-95 Action on NMDARs.
NMDARs bind synaptic scaffolding proteins such as PSD-95 by a PDZ-recognition motif (ESDV) present at the distal end of the C-terminal tails of the NR2A and NR2B subunits (13, 30). To examine whether the effects of PSD-95 on NMDAR trafficking and gating are due to a direct binding interaction between the NR2A subunit and PSD-95, we engineered NR2A subunits lacking the PDZ motif or in which Ser-1462 was replaced by Ala or Glu. The Ser to Glu mutant mimics the negative charge of phosphorylated Ser-1462. Phosphorylation by casein kinase II of the analogous Ser residue in the NR2B subunit disrupts interaction of NMDARs with PSD-95 (25). We reasoned that the Ser to Glu mutation, like the ESDV deletion or Ser1462Ala mutation, should block PSD-95 binding. As above, PSD-95 potentiated basal NMDA-elicited currents and markedly reduced the PKC potentiation in cells expressing wild-type NMDARs (Fig. 6A). Removal of the PDZ-binding motif, or mutation of Ser-1462 to Ala or Glu, abolished PSD-95 potentiation of NMDA currents in confirmation of (17) but did not alter the responses after PKC activation (Fig. 6 and SI Table 2). These findings indicate that Ser-1462 is required for interaction of PSD-95 with NMDARs, and that interaction of the two proteins is required for the increase in NMDA currents induced by PSD-95 expression.
Fig. 6.
Ser-1462 in the ESDV PDZ binding motif of NR2A is required for PSD-95 potentiation. The ESDV motif of NR2A was either deleted (NR2A-ΔESDV) or Ser-1462 was mutated to Ala (NR2A-S1462A) or Glu (NR2A-S1462E). These constructs were coexpressed with NR1–4b, which has the C2′ cassette containing a putative C-terminal PDZ binding motif (tSXV). (A–D) NMDA-elicited currents recorded from oocytes expressing NR1–4b with (A) NR2A wild type, (B) NR2A-ΔESDV, (C) NR2A-S1462A, or (D) NR2A-S1462E in the absence or presence of PSD-95. Mean values of current and PKC potentiation ratios are shown in the bar graphs to the right. (A) As in Figs. 1–5, coexpression of PSD-95 increased the basal responses but did not affect the final current after PKC potentiation, thus, reducing the PKC potentiation ratio. (B–D) All three mutants prevented the increase in response caused by PSD-95 expression and had no effect on PKC potentiation. See SI Table 2 for detailed values. Methods are as in Fig. 1.
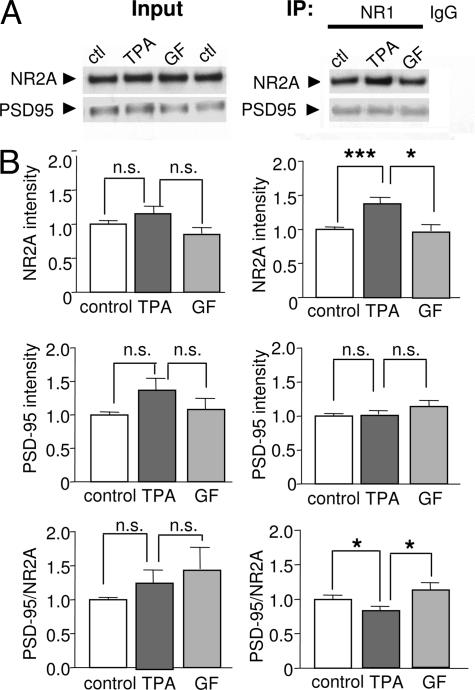
PKC Promotes NR1 and NR2 Assembly, but Does Not Affect Association of PSD-95 with NMDARs in Neurons.
Casein kinase II phosphorylates Ser-1480 in the PDZ-binding motif of the NR2B subunit, thereby disrupting interaction of NMDARs with synaptic scaffolding proteins such as PSD-95 (25). Although PKC does not directly phosphorylate this site in NR2B (25), PKC phosphorylation might directly or indirectly modulate the interaction of NMDARs with PSD-95. To test this possibility, NMDARs were immunoprecipitated with an anti-NR1 antibody from cortical neurons treated with TPA (400 nM with 10 μM okadaic acid, 15 min) or control medium. PKC activation increased the amount of NR2A subunit that coimmunoprecipitated with the NR1 subunit to ≈1.4-fold of control but did not significantly alter the amount of PSD-95 that coimmunoprecipitated with the antibody to either NR1 (Fig. 7) or NR2A (not illustrated). Thus, PKC activation significantly reduced the ratio of PSD-95 to NR2A (control, PSD-95/NR2A = 1.0 ± 0.08, n = 19; TPA, 0.75 ± 0.07, n = 21; P < 0.01 for control vs. TPA; Fig. 7B). Similar results were observed for the NR2B subunit (not illustrated). The TPA-induced increase in NR2A that coimmunoprecipitated with the NR1 antibody was blocked by the highly specific PKC inhibitor GF 109203X (31), verifying TPA action by PKC (Fig. 7B). These findings suggest that PKC activation promotes assembly of NR2 with NR1, consistent with our findings that PKC promotes insertion of new channels in the plasma membrane (Figs. 3 and 4) but has little effect on the interaction of NMDARs with PSD-95.
Fig. 7.
PKC promotes NR1 and NR2 assembly but not NMDAR/PSD-95 association in neurons. (A) Representative Western blots of Input (Left) and immunoprecipitate (IP, Right) probed with an anti-NR1 antibody. Lysates (protein samples) were prepared from cortical neurons at 9 days in vitro. TPA application did not significantly alter total NR2A or PSD-95 protein abundance. TPA increased the abundance of NR2A, but not of PSD-95, that coimmunoprecipitated with an anti-NR1 antibody. TPA effects were blocked by the highly specific PKC blocker GF109203X (GF). (B) Summary of data.
Discussion
Neuronal NMDARs colocalize with PSD-95, a putative NMDAR anchoring protein and core component of the PSD, at excitatory brain synapses (10). PKC increases the number of functional NMDARs at the cell surface of oocytes and neurons and increases NMDA channel opening rate (23). Similarly, PSD-95 promotes insertion of new NMDARs, stabilizes receptors in the plasma membrane, and enhances channel opening rate (17). In each case, the major factor is the increase in channel number. Here we show that, whereas PKC and PSD-95 each independently potentiate NMDAR gating and trafficking, they do so in a nonadditive manner. Importantly, PKC potentiates the NMDA response and surface expression to the same final value in the absence or presence of PSD-95. Thus, PSD-95 coexpression reduces PKC potentiation, defined as the ratio of NMDA elicited current channel opening rate, channel number, or surface expression measured after PKC potentiation to that measured before PKC potentiation. We further show that the PDZ-binding motif in the NR2A subunit, but not that in the C2′ cassette of the NR1 subunit, is required for the effects of PSD-95 on NMDARs and on PKC potentiation. Our coimmunoprecipitation experiments with neurons show that, whereas PKC promotes interaction of NR2A with NR1, it has no effect on interaction of NMDARs with PSD-95 in neurons. A possible mechanism is that there is a limited pool of potential NMDARs, and that PKC promotes assembly and insertion of NMDARs that are not associated with PSD-95.
Findings in the present study are consistent with findings of others that PSD-95 reduces the PKC potentiation ratio of recombinant NMDARs expressed in oocytes (26, 27, 32). The present study extends those observations, in that we show that PKC potentiates NMDAR-elicited currents, channel opening rate, number of functional channels, and surface expression to the same final values in the absence and presence of PSD-95. We compared responses before and after PKC activation in the same cell and compared responses in the absence or presence of PSD-95 in different cells within a given batch of oocytes to reduce variability.
Occlusion of PKC Potentiation by PSD-95 Requires Direct Interaction of PSD-95 with NMDARs.
Deletion or mutation of the PDZ-binding motif in the C terminus of the NR2A subunit abolishes the effect of PSD-95 on NMDA function and surface expression (ref. 17 and the present study) and its occlusion of PKC potentiation (present study). In contrast, the consensus PDZ-binding motif present in the C2′ cassette of the short splice forms of NR1 (30, 33, 34) is not required for the effects of PSD-95 on NMDARs. These findings provide evidence for the specificity of the PSD-95 action by binding to the NR2B subunit. Whereas alternative splicing within the N- and C-terminal domains of the NR1 subunit affects PKC potentiation (ref. 22 and the present study), it does not affect PSD-95 potentiation or apparent occlusion of PKC potentiation. Interestingly, PSD-95 may interact with NR2A- and NR2B-containing receptors by somewhat different but overlapping domains, although the data are incomplete (26, 27). Whereas the first two PDZ domains of PSD-95 are required for inhibition/occlusion of PKC potentiation of NR1-NR2A receptors, the N-terminal domain of PSD-95, which contains potential palmitoylation sites, is required for inhibition of PKC potentiation of NR2B-containing receptors (27). Only the second PDZ domain of PSD-95 is necessary for its inhibitory action on Src-mediated potentiation of channel activity (27). This same palmitoylation domain is required for PSD-95-dependent targeting of NMDARs to synaptic sites (14).
Physiological Significance of the Occlusion.
The present study indicates that PSD-95 increases NMDAR surface expression and channel opening rate and also partially occludes PKC potentiation of NMDAR. Our coimmunoprecipitation experiments are consistent with the possibility that PKC promotes assembly and insertion of new NMDARs that are not associated with PSD-95. The PDZ-binding motifs in the NR2A and NR2B subunits play different roles in the synaptic localization of NMDARs; whereas truncated NR2B subunits lacking the PDZ binding motif cannot localize to the PSD, truncated NR2A subunits can still target to synaptic sites (35). Thus, newly assembled NMDARs would be delivered to different domains within the neuron depending on their NR2 subunit composition.
These findings suggest a mechanism for the activity-dependent regulation of the NMDA excitatory postsynaptic current. Synaptic activity and visual experience modify PSD-95 abundance at synapses (36, 37). The abundance of PSD-95 could, in turn, affect the number of receptors available for insertion in response to activity and thereby affect synaptic plasticity. Our findings in the present study that association of PSD-95 with NMDARs increases NMDAR insertion and opening rate are consistent with findings that after acute knockdown of SAP-102, hippocampal slice cultures from the PSD-95/PSD-93 double knockout exhibit markedly reduced NMDA–excitatory postsynaptic currents (38). Changes in NMDAR number in the postsynaptic membrane provide a critical mechanism for altering synaptic strength rapidly, i.e., over minutes (3). Given that NMDARs, PDZ containing proteins and PKC are widely expressed throughout the central nervous system, modulation of PKC potentiation of synaptic NMDARs by PSD-95 provides a potentially important way to affect synaptic transmission.
Materials and Methods
Selected Stage V and VI oocytes from adult Xenopus were injected with NR1, NR2A, and PSD-95 mRNAs (sources and detailed methods in SI Supporting Text). Whole-cell currents (voltage–clamp) were recorded ≈24 h after injection of PSD-95. Currents were elicited by bath application of NMDA plus glycine as indicated in Mg2+ free frog Ringer solution, normal or with CaCl2 replaced by BaCl2. PKC was activated with the phorbol ester, 12-O-tetradecanoyl phorbol-13-acetate. Single channel currents were recorded from outside-out patches excised from devitellinized oocytes expressing recombinant receptors in the presence or absence of PSD-95. To study the effect of PKC, patches were obtained before and after TPA application. Oocytes expressing NR1–4b/NR2A (NR1100/NR2A) receptors in the absence or presence of PSD-95 were labeled with monoclonal antibody 54.1 directed to the extracellular loop of the NR1 subunit. For coimmunoprecipitation, dissociated cortical neurons were cultured from day 18 rat embryos and harvested in immunoprecipitation buffer after 9–10 days in culture. Extracts were incubated anti-NR1 C-terminal antibody to immunoprecipitate NMDAR complexes. After reaction with Protein G, immunoprecipitates were eluted from beads by incubation with SDS/PAGE loading buffer and subjected to electrophoresis. All data are presented as means ± SEMs for three to six experiments performed with different oocytes from a minimum of two batches. Statistical significance was assessed by Student's unpaired t test.
Acknowledgments
We thank Drs. S. F. Heinemann (The Salk Institute for Biological Studies, San Diego, CA), S. Nakanishi (Osaka Bioscience Institute, Osaka, Japan), and M. Mishina (University of Tokyo, Tokyo, Japan) for providing NR1 and NR2 cDNAs; Dr. M. Sheng (Massachusetts Institute of Technology, Cambridge, MA) for providing PSD-95 cDNA; and Yingjia Liu for technical assistance. This work was supported by National Institutes of Health Grants NS 20752 (to R.S.Z.) and NS 07412 (to M.V.L.B.). M.V.L.B. is the Sylvia and Robert S. Olnick and Distinguished Professor of Neuroscience.
Abbreviations
- NMDAR
NMDA receptor
- PSD
postsynaptic density
- TPA
12-O-tetradecanoyl phorbol-13-acetate
- npo
number of active channels times channel open probability.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0609924104/DC1.
References
- 1.Cull-Candy SG, Brickley S, Farrant M. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 2.Zukin RS, Bennett MVL. Trends Neurosci. 1995;18:306–313. doi: 10.1016/0166-2236(95)93920-s. [DOI] [PubMed] [Google Scholar]
- 3.Carroll RC, Zukin RS. Trends Neurosci. 2002;25:571–577. doi: 10.1016/s0166-2236(02)02272-5. [DOI] [PubMed] [Google Scholar]
- 4.Wenthold RJ, Prybylowski K, Standley S, Sans N, Petralia RS. Annu Rev Pharmacol Toxicol. 2003;43:335–358. doi: 10.1146/annurev.pharmtox.43.100901.135803. [DOI] [PubMed] [Google Scholar]
- 5.Collingridge GL, Isaac JT, Wang YT. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- 6.O'Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL. Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- 7.Friedman HV, Bresler T, Garner CC, Ziv NE. Neuron. 2000;27:57–69. doi: 10.1016/s0896-6273(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 8.Liu XB, Murray KD, Jones EG. J Neurosci. 2004;24:8885–8895. doi: 10.1523/JNEUROSCI.2476-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim E, Sheng M. Nat Rev Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy MB, Beale HC, Carlisle HJ, Washburn LR. Nat Rev Neurosci. 2005;6:423–434. doi: 10.1038/nrn1685. [DOI] [PubMed] [Google Scholar]
- 11.Sans N, Prybylowski K, Petralia RS, Chang K, Wang YX, Racca C, Vicini S, Wenthold RJ. Nat Cell Biol. 2003;5:520–530. doi: 10.1038/ncb990. [DOI] [PubMed] [Google Scholar]
- 12.Sans N, Wang PY, Du Q, Petralia RS, Wang YX, Nakka S, Blumer JB, Macara IG, Wenthold RJ. Nat Cell Biol. 2005;7:1179–1190. doi: 10.1038/ncb1325. [DOI] [PubMed] [Google Scholar]
- 13.Kim E, Cho KO, Rothschild A, Sheng M. Neuron. 1996;17:103–113. doi: 10.1016/s0896-6273(00)80284-6. [DOI] [PubMed] [Google Scholar]
- 14.El Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- 15.Li B, Otsu Y, Murphy TH, Raymond LA. J Neurosci. 2003;23:11244–11254. doi: 10.1523/JNEUROSCI.23-35-11244.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Losi G, Prybylowski K, Fu Z, Luo J, Wenthold RJ, Vicini S. J Physiol (London) 2003;548:21–29. doi: 10.1113/jphysiol.2002.034918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin Y, Skeberdis VA, Francesconi A, Bennett MVL, Zukin RS. J Neurosci. 2004;24:10138–10148. doi: 10.1523/JNEUROSCI.3159-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roche KW, Standley S, McCallum J, Dune LC, Ehlers MD, Wenthold RJ. Nat Neurosci. 2001;4:794–802. doi: 10.1038/90498. [DOI] [PubMed] [Google Scholar]
- 19.Lavezzari G, McCallum J, Dewey CM, Roche KW. J Neurosci. 2004;24:6383–6391. doi: 10.1523/JNEUROSCI.1890-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carroll RC, Beattie EC, von Zastrow M, Malenka RC. Nat Rev Neurosci. 2001;2:315–324. doi: 10.1038/35072500. [DOI] [PubMed] [Google Scholar]
- 21.Fong DK, Rao A, Crump FT, Craig AM. J Neurosci. 2002;22:2153–2164. doi: 10.1523/JNEUROSCI.22-06-02153.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng X, Zhang L, Wang AP, Bennett MVL, Zukin RS. Proc Natl Acad Sci USA. 1999;96:15262–15267. doi: 10.1073/pnas.96.26.15262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lan JY, Skeberdis VA, Jover T, Grooms SY, Lin Y, Araneda RC, Zheng X, Bennett MVL, Zukin RS. Nat Neurosci. 2001;4:382–390. doi: 10.1038/86028. [DOI] [PubMed] [Google Scholar]
- 24.Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. Nat Neurosci. 2002;5:27–33. doi: 10.1038/nn779. [DOI] [PubMed] [Google Scholar]
- 25.Chung HJ, Huang YH, Lau LF, Huganir RL. J Neurosci. 2004;24:10248–10259. doi: 10.1523/JNEUROSCI.0546-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada Y, Chochi Y, Takamiya K, Sobue K, Inui M. J Biol Chem. 1999;274:6647–6652. doi: 10.1074/jbc.274.10.6647. [DOI] [PubMed] [Google Scholar]
- 27.Iwamoto T, Yamada Y, Hori K, Watanabe Y, Sobue K, Inui M. J Neurochem. 2004;89:100–108. doi: 10.1046/j.1471-4159.2003.02293.x. [DOI] [PubMed] [Google Scholar]
- 28.Liao GY, Kreitzer MA, Sweetman BJ, Leonard JP. J Neurochem. 2000;75:282–287. doi: 10.1046/j.1471-4159.2000.0750282.x. [DOI] [PubMed] [Google Scholar]
- 29.Rutter AR, Stephenson FA. J Neurochem. 2000;75:2501–2510. doi: 10.1046/j.1471-4159.2000.0752501.x. [DOI] [PubMed] [Google Scholar]
- 30.Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- 31.Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- 32.Yamada Y, Iwamoto T, Watanabe Y, Sobue K, Inui M. J Neurochem. 2002;81:758–764. doi: 10.1046/j.1471-4159.2002.00886.x. [DOI] [PubMed] [Google Scholar]
- 33.Bassand P, Bernard A, Rafiki A, Gayet D, Khrestchatisky M. Eur J Neurosci. 1999;11:2031–2043. doi: 10.1046/j.1460-9568.1999.00611.x. [DOI] [PubMed] [Google Scholar]
- 34.Standley S, Roche KW, McCallum J, Sans N, Wenthold RJ. Neuron. 2000;28:887–898. doi: 10.1016/s0896-6273(00)00161-6. [DOI] [PubMed] [Google Scholar]
- 35.Prybylowski K, Chang K, Sans N, Kan L, Vicini S, Wenthold RJ. Neuron. 2005;47:845–857. doi: 10.1016/j.neuron.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ehlers MD. Nat Neurosci. 2003;6:231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- 37.Yoshii A, Sheng MH, Constantine-Paton M. Proc Natl Acad Sci USA. 2003;100:1334–1339. doi: 10.1073/pnas.0335785100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elias GM, Funke L, Stein V, Grant SG, Bredt DS, Nicoll RA. Neuron. 2006;52:307–320. doi: 10.1016/j.neuron.2006.09.012. [DOI] [PubMed] [Google Scholar]