Abstract
A critical component of cognitive impairments in schizophrenia can be characterized as a disturbance in cognitive control, or the ability to guide and adjust cognitive processes and behavior flexibly in accordance with one's intentions and goals. Cognitive control impairments in schizophrenia are consistently linked to specific disturbances in prefrontal cortical functioning, but the underlying neurophysiologic mechanisms are not yet well characterized. Synchronous γ-band oscillations have been associated with a wide range of perceptual and cognitive processes, raising the possibility that they may also help entrain prefrontal cortical circuits in the service of cognitive control processes. In the present study, we measured induced γ-band activity during a task that reliably engages cognitive control processes in association with prefrontal cortical activations in imaging studies. We found that higher cognitive control demands were associated with increases in induced γ-band activity in the prefrontal areas of healthy subjects but that control-related modulation of prefrontal γ-band activity was absent in schizophrenia subjects. Disturbances in γ-band activity in patients correlated with illness symptoms, and γ-band activity correlated positively with performance in control subjects but not in schizophrenia patients. Our findings may provide a link between previously reported postmortem abnormalities in thalamofrontocortical circuitry and alterations in prefrontal activity observed in functional neuroimaging studies. They also suggest that deficits in frontal cortical γ-band synchrony may contribute to the cognitive control impairments in schizophrenia.
Keywords: cognition, γ oscillations, prefrontal cortex
Cognitive impairments are a core feature of schizophrenia and one of the strongest predictors of functional outcome in this debilitating disorder (1). One critical set of deficits can be characterized as a disturbance in cognitive control, or the ability to guide and adjust cognitive processes and behavior flexibly in accordance with one's intentions and goals. As shown in functional magnetic resonance imaging (fMRI) studies, cognitive control processes are supported by the dorsolateral prefrontal cortex (DLPFC) (2–4), and they are impaired in schizophrenia in association with disturbances in DLPFC activity (5–7). However, although disturbed PFC activity and cognition in schizophrenia are well established, little is known about the nature of the underlying neurophysiologic disturbances.
Studies of the fMRI blood oxygen level-dependent (BOLD) signal suggest that high-frequency localized neuronal synchrony drives BOLD responses (8), raising the possibility that disturbances in high-frequency neural synchrony may give rise to the impaired prefrontal cortical activations observed in fMRI studies in schizophrenia. This notion is supported by postmortem work in schizophrenia showing that chandelier cell interneurons, which have the anatomic and electrophysiologic properties to coordinate fast oscillations in pyramidal neurons, are functionally impaired in the disorder (9). These findings suggest that fMRI findings of disturbed PFC function may be caused by an alteration in high-frequency synchronous oscillations.
Synchronization of cortical neuronal activity in the γ band (30–80 Hz) appears to be important for a wide range of perceptual and cognitive processes. Studies of perception show that visual stimuli can elicit γ-band synchrony in animals (10, 11) and humans (12, 13), and such synchronous γ-band activity may be critical for perceptual feature binding (14). Moreover, findings of γ-band synchrony extend to higher cognitive processes, such as associative learning (15) and working memory (16, 17). That γ-band synchrony is found in association with a wide variety of perceptual and cognitive functions suggests that it may be a generalized mechanism for entraining networks of cortical neurons. By extension, γ-band synchrony may also subserve the coordination of PFC networks that subserve cognitive control.
If frontal cortical γ-band oscillations are necessary for cognitive control, then disturbances in the γ-band activity may lead to the impaired cognitive control observed in schizophrenia. Some studies in schizophrenia have found disturbances in steady-state activity (18), which refers to neural oscillations that are entrained to an oscillating stimulus, whereas others found disturbed evoked γ activity that derives from oscillations that are phase-locked to presented stimuli (19, 20). Spencer and colleagues (21, 22) examined phase-locking to both stimuli and responses.
However, it is not clear how findings concerning stimulus-locked responses are related to impaired cognitive control in schizophrenia. Higher-order cognitive processes are thought to be more commonly associated with induced oscillatory activity (23) or oscillations that are not phase-locked to the stimulus. In the present study, we thus measured induced γ-band activity during the Preparing to Overcome Prepotency task, a paradigm that engages cognitive control and reliably elicits prefrontal cortical activation in healthy subjects and comparably less prefrontal cortical activity in schizophrenia (7). Accordingly, we hypothesized that increases in control demands would elicit increases in prefrontal induced γ-band activity in healthy subjects but that this modulation of this activity would be reduced in schizophrenia subjects.
Results
Cognitive Task Description.
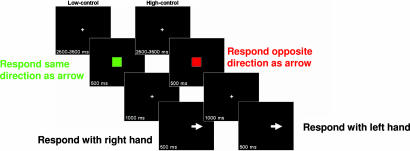
For a description of the cognitive task, see Fig. 1.
Fig. 1.
Cognitive task description. (Left) Low-control trials signaled by green cues. (Right) High-control trials signaled by red cues.
Behavioral Performance.
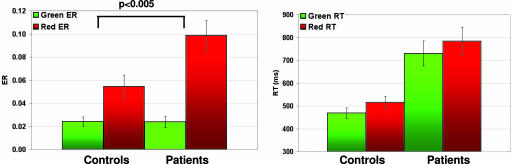
Performance was compared (Fig. 2) by using two-way repeated measures ANOVAs with diagnostic group (schizophrenia vs. healthy control group) as a between-subject factor and condition (high- vs. low-control) as a within-subject factor. For error rates, this analysis revealed the main effects of condition (F = 10.71, P < 0.001) but not diagnostic group (F = 3.71, P = 0.63), with a significant diagnostic group × condition interaction (F = 10.18, P < 0.005). Planned comparisons of the condition effect (high-control minus low-control error rates) between groups revealed that this interaction was driven by higher-condition effects in the patient vs. control group (t = 2.04, P < 0.005, two-tailed). For reaction times, there were main effects of diagnostic group (F = 0.59, P < 0.005) and condition (F = 17.47, P < 0.001). There was no group × condition interaction (F = 0.89, P = 0.35).
Fig. 2.
Behavioral data. Error rates (ER) and reaction times (RT) for low-control (green) and high-control (red) trials are shown. Patients show greater differences between high- and low-control error rates compared with controls.
Electroencephalogram (EEG).
Induced γ-band oscillations.
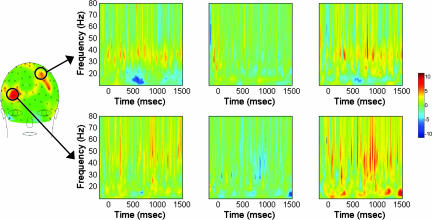
To test our hypothesis that increased induced frontal γ-band activity would be associated with cognitive control, we assessed induced γ over the delay period (500–1,500 ms from cue onset), comparing high-control (respond opposite to direction indicated by upcoming probe stimulus) vs. a low-control (respond in same direction indicated by probe) trials. In comparing induced γ-band activity for controls vs. schizophrenia subjects, two electrodes, both in frontal areas (Fig. 3), were significantly higher for high- vs. low-control trials (both at P < 0.05), one in a right frontal region (electrode 2 or AF8 in the 10/10 system; for conversion table, see ref. 24) and the other in a left frontal region (electrode 21 or FC1 in the 10/10 system).
Fig. 3.
Frequency–time plots of within- and across-group comparisons of induced γ-band activity. All plots span frequencies 8–80 Hz (y axis), which includes the 30- to 80-Hz range used to define γ activity in the band-specific analyses, over the baseline (−200 to 0 ms), cue (0–500 ms), and delay (500–1,500 ms) periods (x axis). (Left and Center) Statistical comparisons (Wilcoxon signed-rank test) of high- vs. low-control conditions. (Left) Controls. (Center) Patients. (Right) Group comparison of the difference between the high- and low-control conditions (controls vs. patients; Mann–Whitney U test). Shown are negative log P values for all plots. For display purposes, all values represent the statistical comparisons without multiple-comparison corrections. (Upper) Left frontal electrode. (Lower) Right frontal electrode. The frontal scalp map is a summary map of group differences over the delay period, showing locations of the left and right electrodes (circled; the back of the head is not shown because of very minimal activation differences). (Left and Center) Hot colors denote greater γ power for the high- vs. low-control condition, and cool colors show the reverse. (Right) Hot colors denote controls having greater positive differences between the high- and low-control conditions compared with patients, and cool colors show the reverse.
To rule out group differences in induced γ-band activity being driven by underlying evoked γ activity, we examined evoked activity for the significant right and left frontal electrodes. Neither electrode demonstrated significant within or between group (right frontal, P = 0.79; left frontal, P = 0.73) differences in evoked γ activity. As a more direct verification that induced γ was specifically indexed, induced γ analyses were repeated with evoked γ averages for each subject subtracted from the averaged per-trial wavelet-transformed data (25). Both frontal electrodes retained significance (at P < 0.05).
Induced α- and θ-band oscillations.
To examine the specificity of our induced γ findings and rule out broader spectrum increases, induced θ and α activity was examined. In the θ band, although controls showed no significant modulation by condition, group comparisons showed patients with greater modulation by condition for three electrodes (all P < 0.05), in the parietoocciptal area: electrodes 73, 92, and 95 (Oz, P8, and close to PO8, respectively, in 10/10). In α-band analyses, both groups tended to show decreases in α-band activity in frontal areas with increased control, and the group comparison revealed that patients showed relatively greater decreases (P < 0.05) in α in the right frontal region (electrode 3, AF4 in 10/10).
To complement the θ- and α-band analyses in assessing the possibility of broad-spectrum modulations, a wavelet transform across a broad range of frequencies (8–80 Hz) was performed for the right and left frontal electrodes that were significant for the across-group contrasts for γ-band activity (Fig. 3). The scalp map shows that right and left frontal regions exhibited group differences but that only one electrode in each region (nonhomologous sites) achieved significance (marked by circles). In both frontal regions, there were increased modulations in controls vs. patients in the lower-γ-frequency band centered at ≈45 Hz and 37 Hz in the right and left electrodes, respectively. These modulations start during the cue, extending into the delay period, and are apparent in the within-group contrasts for the controls but not for the patients. In the right frontal electrode, there are also significant differences in the α and lower-β frequency range between controls and patients over much of the delay period. These differences over the delay period were the result of both modest increases in controls as well as decreases in patients, especially at the end of the delay period. The decrease in α activity by increased control demand for patients is consistent with similar decreases observed for electrode 3 (AF4), which is directly adjacent to this right frontal electrode. Plots of the power for each of the groups and conditions for the two frontal electrodes [see supporting information (SI) Fig. 5] indicate what gave rise to the within and between group statistics. The left electrode had greater modulation of γ-band activity by condition (high vs. low control) for controls compared with patients. For the right frontal electrode, patients had overall greater power in frequencies higher than ≈25 Hz in both conditions. However, the patients lacked the modulation by condition that the controls exhibited.
Correlations of γ-band activity with clinical symptoms.
Among the symptom clusters of schizophrenia, Disorganization has been shown to be most strongly negatively correlated with both behavior (26, 27) and DLPFC activation (6). We probed for such correlations between γ-band activity in electrodes 2 (AF8) and 21 (FC1) over the early, middle, and late delay periods, and symptom scores for Disorganization, as well as Reality Distortion and Poverty symptoms. For electrode 2 (AF8), the only significant finding was a positive correlation between middle delay γ-band activity and Reality Distortion (r = 0.47, t = 1.9, P < 0.05, one-tailed). This correlation seemed largely driven by one influential point, which, when excluded, made the correlation nonsignificant (r = 0.22, t = 0.80, P = 0.22, one-tailed). The largest correlations with Poverty symptoms and Disorganization over any part of the delay were r = 0.19 and r = 0.04, respectively. For electrode 21 (FC1), the only significant result was an inverse correlation for late delay γ and Disorganization (r = −0.45, t = −1.8, P < 0.05, one-tailed). The largest correlations with Reality Distortion and Poverty symptoms over any part of the delay were r = −0.29 and r = 0.23, respectively.
Correlations of γ-band activity with behavioral performance.
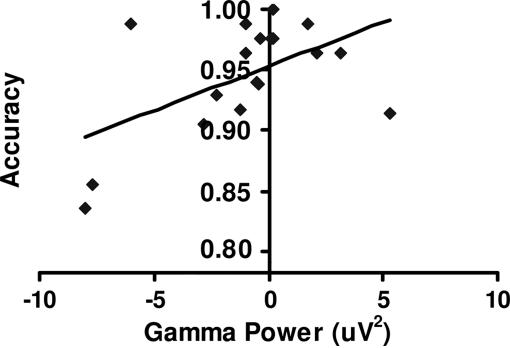
To examine whether γ-band activity would be predictive of behavior, we performed correlations between γ-band activity in electrodes 2 (AF8) and 21 (FC1) during the early, middle, and late delay periods, and performance during the high-control condition (separately for patients and controls). For electrode 2 (AF8), controls showed a relatively high correlation (r = 0.54, t = 2.6, P = 0.01) between late delay γ-band activity and accuracy (Fig. 4). For electrode 21 (FC1), controls showed a nearly significant correlation between late delay γ-band activity accuracy (r = 0.38, t = 1.7, P = 0.057). Controls demonstrated no significant correlations with reaction times for either electrode. Patients exhibited no significant correlations between γ-band activity and performance for either electrode. These correlations with performance were repeated for low-control related γ-band activity with no significant findings for controls or patients. To rule out the possibility that correlations between Disorganization and γ-band activity in electrode 21 (FC1) were obscuring a relationship between performance and γ activity in patients, this latter correlation was redone, partialing out the effect of Disorganization. The resulting correlation between accuracy and late delay γ activity increased from r = 0.07 to 0.31, but it remained nonsignificant (t = 1.18, P = 0.13, one-tailed).
Fig. 4.
Correlation with performance. Shown are accuracy rates for high-control condition for healthy control subjects plotted against late delay γ power for the high-control condition in the right frontal region.
Discussion
γ-Band synchronous oscillations have been associated with a wide range of perceptual and cognitive processes. In this study, increases in cognitive control demands were associated with increases in induced γ activity over prefrontal cortical areas for healthy controls. This modulation of frontal cortical γ was diminished in schizophrenia subjects, with disturbances in γ-band activity correlating with their Disorganization symptoms. Further, increases in γ-band activity correlated with performance in controls but not patients. These findings are consistent with the proposal that disturbances in frontal induced γ synchrony¶ may underlie the cognitive control deficits observed in this disorder.
The modulation of induced prefrontal γ delay period activity in association with cognitive control demands in controls complements previous EEG findings of working memory maintenance-related γ-band activity (16, 17). Our findings suggest that synchronous γ-cortical oscillations may support not only basic object representations but also more abstract representations involved in cognitive control, e.g., those that mediate stimulus–response reversals that suppress prepotent tendencies. That prefrontal γ activity has a role in mediating top-down control is suggested by the finding that increases in right frontal delay activity are associated with performance during trials requiring higher control, consistent with previous findings relating increased delay-related DLPFC activations and decreased interference (3).
Schizophrenia subjects failed to show similar modulations of prefrontal γ by control demands, both in the condition-related contrasts and correlations with performance. These results are consistent with our fMRI study of schizophrenia subjects using the Preparing to Overcome Prepotency task, which also showed reduced DLPFC modulation by control demands and a correlation between DLPFC activation and performance in healthy controls but not patients (7). In the current study, patients exhibited a variable pattern of induced γ-band activity across the PFC (higher on the right, lower on the left), analogous to similar variability in fMRI studies of PFC function in schizophrenia (28), but the consistent finding was a lack of modulation of induced γ by cognitive control demands. Although patients did not exhibit correlations between γ-band activity and performance, correlations with symptoms suggested a relationship between γ-band disturbances and clinical presentation. Left prefrontal γ-band activity was negatively correlated with Disorganization symptoms but not Reality Distortion or Poverty symptoms, consistent with similar correlations between left DLPFC activation and Disorganization in previous studies (6, 7). Right prefrontal γ activity correlated with Reality Distortion, consistent with similar reports in the literature (29). However, this correlation depended heavily on one influential point, and therefore it requires replication. Together, these findings suggest that disturbed modulation of prefrontal cortical γ synchrony in subjects may underlie cognitive control disturbances as well as possibly contributing to clinical symptoms.
The specificity of our findings of induced γ-band activity in prefrontal regions for controls and schizophrenia subjects was supported by a number of findings. First, analyses of evoked γ showed that findings in the induced γ responses were not confounded by evoked activity. Second, the θ- and α-bands did not exhibit the same patterns of activity that were observed in the γ-band, ruling out broad-spectrum modulations. α-Band analysis showed greater decreases for high-control trials, consistent with similar findings (30) for both controls and patients, with patients showing greater such modulation in a right frontal region. The lack of overlap in the directionality of α-band and γ-band findings is consistent with a report of an absence of any spatial or temporal correlations between α- and γ-band activity (31). Although Gevins and colleagues (30) have shown frontal midline θ-band activity modulating with memory load in healthy subjects, our control subjects did not exhibit significant θ-band activity, perhaps because of equal working memory demands across the two conditions. Patients, however, did exhibit increased modulation in the parietooccipital region compared with controls. The significance of greater modulations for patients in the θ and α bands is unclear, but it may point to compensatory mechanisms in the absence of proper entrainment of frontal γ activity.
Although our findings of disturbed prefrontal-induced γ-band activity in schizophrenia are consistent with our previous fMRI studies that used the same or similar tasks (5–7), there are some possible limitations to the current study. First, the patient data were possibly confounded by medication effects and chronicity. We are currently studying first-episode, medication-naïve schizophrenia subjects to address these possible confounds. Another possibility is that the stimulus properties of the cues, namely, the colors that indicated the low- vs. high-control trials (green vs. red, respectively; cues were matched for size and luminance) may have had differential effects across groups. Previous reports (32) have noted increased modulation of induced γ-synchrony in monkey visual cortex by red over green stimuli. However, these responses quickly dissipated after stimulus offset, suggesting that these “bottom-up” influences would not explain our results over the whole delay period. Further, all of the spectral analyses were confined to correct trials to ensure that subjects were on task, implying that, whatever more perceptually driven processes were occurring, subjects were properly interpreting the cues. Future studies that counterbalance cue color across subjects will more definitively answer this issue.
Our findings of disturbed γ-band synchrony in schizophrenia are consistent with neurobiological findings in this disorder. Jones (33) proposed that observed anatomical evidence for functional disturbances in thalamocortical circuits in schizophrenia first reported in his laboratory could lead to impaired thalamocortical oscillations in schizophrenia. Clearly, disturbances in either the thalamocortical and/or corticothalamic projections could result in disruptions in γ-band thalamocortical oscillations. Reduced numbers of thalamocortical projection neurons have been reported (34, 35) as well as functional impairments in corticothalamic projections (36). One intriguing possibility is that such disturbances in corticothalamic projections would lead not only to disturbed γ-band oscillations but also to increased θ-band activity as was found in the schizophrenia subjects of this study. Llinas and colleagues (37) proposed that increased inhibition or deafferentation of the thalamic relay cells could lead to aberrant increases in θ rhythms. Specifically, they proposed that the hyperpolarized state of relay neurons induced by deafferentation or inhibition leads to periodic bursting at θ frequency through deinactivation of T-type calcium channels and feedback inhibition by thalamic reticular nucleus neurons. This relay neuron hyperpolarization-dependent cycle gives rise to θ rhythms during normal physiologic states such as sleep, but it is proposed to occur pathologically in the context of neuropsychiatric disorders, including schizophrenia. Another pathophysiologic mechanism that implicates prefrontal cortical neurons is described by Lewis and colleagues (38), who underscore the possible critical role of functional disturbances in interneurons, the chandelier class of GABA cells being one of the more consistently implicated. Each chandelier cell exhibits fast-spiking, nonadapting firing patterns, and it forms synapses onto the axon initial segments (near the site of action potential generation) of up to 300 pyramidal cells (39), and it is thus enabled with both distinctive electrophysiologic properties and strategic synaptic locations to modulate the electrical outflow of a large number of pyramidal cells powerfully. Consequently, disturbances in chandelier cell functioning could impair the ability of cortical circuits to engage in high-frequency synchronous oscillations, either locally or in coordination with the thalamus, consistent with the reduced γ-band synchrony observed for patients in the current study. Interestingly, glutamate receptor hypofunction, a pathophysiologic feature of schizophrenia (40), may lead to chandelier cell disturbances as a downstream consequence (41). Thus, possibly related disturbances in both excitatory and inhibitory neurotransmission may lead to disturbed γ-band synchrony in schizophrenia.
In summary, we have demonstrated an association between prefrontal cortical γ-band activity and cognitive control processes that is present in healthy controls but absent in schizophrenia subjects. Prefrontal γ-band activity was related to performance in controls with suggestive correlations between disturbances in γ activity and symptoms in patients. Although fMRI studies have shown prefrontal disturbances in schizophrenia in association with cognitive control impairments, this study provides EEG evidence with improved temporal resolution and suggestive evidence that disturbed γ synchrony may underlie the decreased activations observed in imaging studies. EEG assessments of prefrontal γ synchrony, then, may provide a useful tool for assessing impairment of prefrontal cortical circuits in tandem with behavioral measures of cognitive control disturbances in schizophrenia. Assessments of γ synchrony may also help in assessing the efficacy of therapeutic agents that aim at improving the functional integrity of cortical circuits that subserve such coordinated activity.
Materials and Methods
Subjects.
Eighteen healthy participants (17 right-handed) and 15 schizophrenia/schizoaffective disorder participants (14 right-handed) participated in this study. Exclusion criteria consisted of a lifetime history of seizures or significant head trauma, mental retardation, substance use or abuse within the previous 6 months, use of benzodiazepine or other anticonvulsants. Written informed consent was completed before testing in accordance with the Institutional Review Board at the University of Pittsburgh; participants were monetarily compensated when they completed the study. All participants were 20–50 years old. Participants were clinically stable, medicated outpatients with schizophrenia or schizoaffective disorder, and they were recruited from a large, metropolitan psychiatric hospital (Western Psychiatric Institute and Clinic, Pittsburgh, PA). Diagnoses were based on Structured Clinical Interview for DSM-III-R (42), and we used DSM-IV criteria for schizophrenia. Summary clinical ratings scores were derived [following Barch et al. (ref. 26)] for Reality Distortion, Disorganization, and Poverty Symptoms from the Brief Psychiatric Rating Scale [BPRS (ref. 43)] and the Scales for the Assessment of both Positive and Negative Symptoms [SANS (ref. 44); SAPS (ref. 45)]. Patients' average ratings (mean ±SD) were the following: Reality Distortion, 2.55 ± 0.84; Disorganization, 1.77 ± 0.60; Poverty Symptoms, 2.54 ± 0.52. Healthy controls were recruited through hospital and community advertisements. Controls were interviewed by using the nonpatient version of the Structured Clinical Interview for DSM-III-R (46), and they were excluded for any lifetime history of an Axis I disorder as well as a first-degree family history of psychotic disorders. Comparisons made using t tests determined that there were no significant differences between controls and patients in the demographic variables of age [patients, 37.1 ± 9.0 years (SD); controls, 36 ± 6.2 years], gender (patients, 9 males; controls, 10 males), and parental education (patients, 14.0 ± 3.5 years; controls, 13.0 ± 3.4 years).
Procedure.
Task.
Stimuli were presented centrally on a computer monitor by using E-Prime (Psychological Software Tools, Pittsburgh, PA). Trials proceeded in the following order (see Fig. 1): cue (a green or red square; visual angle, 5.0°), delay period, probe (a white arrow pointing left or right; visual angle, 1.6° × 1.3°), and a variable intertrial interval. Cues signaled conditions requiring low vs. high degrees of cognitive control (referred to as the low- and high-control conditions, respectively). Over the delay, subjects were required to maintain the trial-type information and to prepare for a response to the upcoming probe. For the low-control condition (green cues), subjects were required to respond in the direction of the arrow that followed (e.g., for a right-pointing arrow, press the right button). For the high-control condition (red cues), subjects were required to respond in the opposite direction (e.g., for a right-pointing arrow, press the left button). For responses, subjects pressed the left button on a button box with their left index finger, and the right button with their right index finger. Cues were matched for luminance. To reinforce the prepotency of the stimulus–response mappings of the low-control trials, thereby increasing the control requirements during the high-control trials, the majority (75%) of the trials were low-control with the remaining 25% high-control. Both the cue and probe stimuli had durations of 500 ms. The delay period was fixed at 1,000 ms, during which subjects were required to maintain fixation on a fixation cross presented centrally on the screen. Intertrial intervals were randomized between 2,500 and 3,500 ms. Participants received 14 blocks of 24 trials each. Before experimentation, subjects practiced the task until at least 90% accuracy was attained. For most subjects, one practice block (24 trials) was sufficient.
Recordings.
EEG data were acquired by using a 129 Ag-AgCl-coated carbon fiber electrode Geodesic Sensor Net (EGI, Eugene, OR) with a sampling frequency of 250 Hz. Data were filtered online with a 0.1- to 100-Hz bandpass hardware filter. Electrode impedances were kept at <50 kΩ. All channels were referenced to Cz.
Offline processing.
For the γ- and α-band analyses, data were filtered offline by using an 8- to 100-Hz bandpass, 60-Hz notch Butterworth Filter (time constant, 0.0199s; slope, 12 dB/oct). For θ-band analyses, data were filtered offline by using a 2- to 15-Hz bandpass. Epochs were defined as −400 to +1,700 ms relative to the cue onset. Error trials and epochs containing artifacts (EEG or electrooculogram exceeding ±100 μV) were excluded. Data were re-referenced against the average reference (47).
Time–frequency transformation of the data.
Time–frequency analyses were carried out by using Brain Vision Analyzer (Brain Products GmbH, Munich, Germany). The wavelet transformation was applied by using the complex Morlet wavelet, which is defined by
which describes a family of functions that oscillates according to the frequency parameter ω0 with a Gaussian envelope exp(−x2/2) and a constant (c = 3.8) that appropriately normalizes the integral of the wavelet and determines the number of cycles present in each wavelets, which remains constant across all frequency bands (thus, lower frequency band wavelets will extend across a longer time interval).
Wavelet analyses decomposed the signal between 8 and 100 Hz (40 frequency steps) into its time–frequency components. Frequency subbands, labeled “scales,” are defined by a lower and upper boundary as well as a central frequency. Thus, scales number from 1 through 40, going from lower to higher frequencies, respectively. Because the parameter c determines the number of cycles, it also determines how wide the each scale will be for the different frequencies. For the γ- and α-band analyses, power values from scale 31 (27.91- to 83.74-Hz bandwidth, 55.83-Hz central frequency ‖) and scale 2 (8.09- to 12.63-Hz bandwidth, 10.36-Hz central frequency) were extracted, respectively. For θ-band analyses, the data were bandpass-filtered (2–15 Hz) to include the θ-band. Scale 5 (4.94- to 7.72-Hz bandwidth, 6.33 central frequency) was extracted to approximate θ-band. Data were referenced to a −200 to −50 ms precue baseline interval for the γ-band data and to a −300 to −50 ms precue interval for the θ- and α-bands because of the larger periods for these latter two frequency bands. Induced responses are not time-locked to the stimulus, and they are determined by averaging the segments after they have been wavelet-transformed. For evoked responses, which are time-locked to the stimulus, data were first averaged for each condition (before wavelet transformation), with the resulting averages then being wavelet-transformed.
Data Analysis.
Based on a criterion requiring 30 or more artifact-free trials per condition, 5 patients were excluded from the analysis (leaving 15 included in the analyses); all control subjects were included. Controls averaged 194 low-control trials and 64 high-control trials. Patients averaged 148 low-control and 50 high-control trials. Because normality could not be assumed of the spectral power distributions for each condition at each time point, statistical contrasts of the high- vs. low-control conditions were carried out by performing the nonparametric Wilcoxon signed-ranks test for matched pairs for within-group contrasts and Mann–Whitney U test for unmatched pairs for across-group comparisons. The α error was of concern because of the number of multiple comparisons across electrodes and time points. To address this issue for the number of time points, the following correction was used, involving an empirically derived estimate of the type I error. For each of the three frequency bands (γ, θ, and α) on a per-electrode basis, we segmented the data into 20 nonoverlapping bins of 48 ms each, and we limited analysis to the delay period because this was the interval most relevant to the hypothesis.
Such data reduction drastically reduces the number of comparisons. However, recognizing that even 20 comparisons is relatively large, we derived empirical thresholds for significance for each electrode by randomly permuting the data 1,000 times and determining the number of bins that would have to be significant by the standard uncorrected test, to be above chance level (α = 0.05). The thresholds involved the vast majority of electrodes having >6, 8, or 10 significant bins (of a possible total of 20) for the γ-, α-, and θ-band data, respectively. Deriving thresholds in this manner helped accommodate any variability in the noise characteristics across the electrode–frequency combinations. All significant results reported in the text are based on statistical tests with multiple-comparisons correction. For plotting purposes only (Fig. 3), P values have been negative log-transformed. All negative log P values shown in figures are derived from uncorrected P values.
For the γ-band analyses, any significant frontal electrodes were further examined by inspecting the γ-band power averages for the high vs. low conditions, respectively. Also, to ensure that any modulations in the induced γ responses were not actually the result of evoked activity incorporated into the averages, we examined both evoked averages as well as performing additional analyses by using the induced averages as described above, but with the evoked averages subtracted for each subject (25). Further, on each of these electrodes, we conducted statistical tests across time–frequency combinations that spanned the full cue-delay interval and frequency bands that spanned 8–80 Hz (in 37 logarithmic increments). For within-group contrasts of the high- vs. low-control condition γ activity, Wilcoxon signed-ranks test for matched pairs was used, with Mann–Whitney U test for unmatched pairs for across-group comparisons.
Correlations of γ-Band Activity with Clinical Symptoms and Behavioral Performance.
To assess the clinical and behavioral relevance of any group differences in γ-band activity, significant electrodes in the group contrast were submitted to correlation analyses, probing for any association with symptoms and performance.
Previous studies have shown that both poorer behavioral performance (26) as well as decreased DLPFC activation (6) during task trials requiring higher degrees of cognitive control are significantly and most strongly associated with the Disorganization cluster of symptoms (48) in schizophrenia subjects. To check for similar associations, we performed correlations between γ-band activity over the delay period and symptom scores for Reality Distortion, Disorganization, and Poverty Symptoms. Given the temporally smoothed nature of fMRI BOLD, it is difficult to know at the temporal resolution of EEG which portion of the delay period might give rise to the DLPFC activation correlations with Disorganization symptoms. We therefore divided the delay period into three bins, referenced with respect to cue stimulus offset: early (0–332 ms), middle (333–664 ms), and late (665–996 ms). The γ activity in each of these bins was correlated with the symptom scores across schizophrenia subjects.
Similarly, previous reports of associations between DLPFC activations and cognitive control (3) motivated checking for similar correlations between γ-band activity during the early, middle, or late delay period (as defined above) and performance during the high-control trials. Correlations were performed separately for controls and patients.
Supplementary Material
Acknowledgments
We thank Joseph M. Orr for help with graphics and data processing. This work was supported by National Institute of Mental Health Grant MH64190 and a Translational Clinical Scientist Award from the Burroughs–Wellcome Foundation (to C.S.C.) and a National Alliance for Research on Schizophrenia and Depression Young Investigator Award (to R.Y.C.).
Abbreviations
- BOLD
blood oxygen level-dependent
- PFC
prefrontal cortex
- DLPFC
dorsolateral PFC
- EEG
electroencephalogram
- fMRI
functional magnetic resonance imaging.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0609440103/DC1.
“Synchrony” can refer to “phase synchrony” that describes consistent phase relationships with respect to a stimulus or response. In this context, however, we refer to the fact that for increases in induced γ band power, clustered, similarly oriented groups of neurons must fire at the same time with respect to each other on any given trial. These requirements are true for any detectable scalp EEG signal, as with regular event-related potentials, but in this case, the synchronous activations modulate in an oscillatory manner.
This central frequency is close to that of line noise (60 Hz). Although a 60-Hz notch filter was used, imperfect filtering could lead to spurious results. As an extra check, for the significant electrodes for the γ band analysis, data were reanalyzed by using a lower sub-band of γ (38–48 Hz, central frequency of 43 Hz). The frontal electrodes retained significance, which is perhaps not surprising given that most of the condition and group related differences were seen in the lower range of γ (see Fig. 3).
References
- 1.Green MF. Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 2.Kerns JG, Cohen JD, MacDonald AW, III, Cho RY, Stenger VA, Carter CS. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald AW, III, Cohen JD, Stenger VA, Carter CS. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 4.Miller EK, Cohen JD. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 5.Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald A, III, Noll DC, Cohen JD. Arch Gen Psychiatry. 2001;58:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- 6.MacDonald AW, III, Carter CS, Kerns JG, Ursu S, Barch DM, Holmes AJ, Stenger VA, Cohen JD. Am J Psychiatry. 2005;162:475–484. doi: 10.1176/appi.ajp.162.3.475. [DOI] [PubMed] [Google Scholar]
- 7.Snitz BE, MacDonald A, III, Cohen JD, Cho RY, Becker T, Carter CS. Am J Psychiatry. 2005;162:2322–2329. doi: 10.1176/appi.ajp.162.12.2322. [DOI] [PubMed] [Google Scholar]
- 8.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 9.Lewis DA. Brain Res Brain Res Rev. 2000;31:270–276. doi: 10.1016/s0165-0173(99)00042-9. [DOI] [PubMed] [Google Scholar]
- 10.Eckhorn R, Bauer R, Jordan W, Brosch M, Kruse W, Munk M, Reitboeck HJ. Biol Cybern. 1988;60:121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- 11.Gray CM, Singer W. Proc Natl Acad Sci USA. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller MM, Bosch J, Elbert T, Kreiter A, Sosa MV, Sosa PV, Rockstroh B. Exp Brain Res. 1996;112:96–102. doi: 10.1007/BF00227182. [DOI] [PubMed] [Google Scholar]
- 13.Lutzenberger W, Pulvermuller F, Elbert T, Birbaumer N. Neurosci Lett. 1995;183:39–42. doi: 10.1016/0304-3940(94)11109-v. [DOI] [PubMed] [Google Scholar]
- 14.Singer W, Gray CM. Annu Rev Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- 15.Miltner WHR, Braun C, Arnold M, Witte H, Taub E. Nature. 1999;397:434–436. doi: 10.1038/17126. [DOI] [PubMed] [Google Scholar]
- 16.Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, Schulze-Bonhage A, Kahana MJ. Cerebral Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- 17.Tallon-Baudry C, Kreiter A, Bertrand O. Visual Neurosci. 1999;16:449–459. doi: 10.1017/s0952523899163065. [DOI] [PubMed] [Google Scholar]
- 18.Kwon JS, O'Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, Hasselmo ME, Potts GF, Shenton ME, McCarley RW. Arch Gen Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallinat J, Winterer G, Herrmann CS, Senkowski D. Clin Neurophysiol. 2004;115:1863–1874. doi: 10.1016/j.clinph.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Hong LE, Summerfelt A, McMahon R, Adami H, Francis G, Elliott A, Buchanan RW, Thaker GK. Schizophr Res. 2004;70:293–302. doi: 10.1016/j.schres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. J Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, Shenton ME, McCarley RW. Proc Natl Acad Sci USA. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tallon-Baudry C, Bertrand O. Trends Cognit Sci. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- 24.Luu P, Ferree T. Determination of the Geodesic Sensor Nets' Electrode Positions and Their 10-10 International Equivalents. Eugene, OR: Electrical Geodesics, Inc; 2000. [Google Scholar]
- 25.Luu P, Tucker DM, Makeig S. Clin Neurophysiol. 2004;115:1821–1835. doi: 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 26.Barch DM, Carter CS, MacDonald AW, III, Braver TS, Cohen JD. J Abnorm Psychol. 2003;112:132–143. [PubMed] [Google Scholar]
- 27.Cohen JD, Barch DM, Carter CS, Servan-Schreiber D. J Abnorm Psychol. 1999;108:120–133. doi: 10.1037//0021-843x.108.1.120. [DOI] [PubMed] [Google Scholar]
- 28.Manoach DS. Schizophr Res. 2003;60:285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- 29.Lee K, Williams L, Haig A, Gordon E. Cognit Neuropsychiatry. 2003;8:57–71. doi: 10.1080/713752240. [DOI] [PubMed] [Google Scholar]
- 30.Gevins A, Smith ME, McEvoy L, Yu D. Cerebral Cortex. 1997;7:374–385. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- 31.Muller MM, Junghofer M, Elbert T, Rochstroh B. Neuroreport. 1997;8:2575–2579. doi: 10.1097/00001756-199707280-00031. [DOI] [PubMed] [Google Scholar]
- 32.Rols G, Tallon-Baudry C, Girard P, Bertrand O, Bullier J. Visual Neurosci. 2001;18:527–540. doi: 10.1017/s0952523801184038. [DOI] [PubMed] [Google Scholar]
- 33.Jones EG. Schizophr Bull. 1997;23:483–501. doi: 10.1093/schbul/23.3.483. [DOI] [PubMed] [Google Scholar]
- 34.Danos P, Baumann B, Bernstein HG, Franz M, Stauch R, Northoff G, Krell D, Falkai P, Bogerts B. Psychiatry Res. 1998;82:1–10. doi: 10.1016/s0925-4927(97)00071-1. [DOI] [PubMed] [Google Scholar]
- 35.Popken GJ, Bunney WE, Jr, Potkin SG, Jones EG. Proc Natl Acad Sci USA. 2000;97:9276–9280. doi: 10.1073/pnas.150243397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, Lewis DA. Am J Psychiatry. 1999;156:1580–1589. doi: 10.1176/ajp.156.10.1580. [DOI] [PubMed] [Google Scholar]
- 37.Llinas RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Proc Natl Acad Sci USA. 1999;96:15222–15227. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis D, Pierri J, Volk D, Melchitzky D, Woo T-U. Biol Psychiatry. 1999;46:616–626. doi: 10.1016/s0006-3223(99)00061-x. [DOI] [PubMed] [Google Scholar]
- 39.Lewis DA, Volk DW, Hashimoto T. Psychopharmacology. 2004;174:143–150. doi: 10.1007/s00213-003-1673-x. [DOI] [PubMed] [Google Scholar]
- 40.Moghaddam B. Neuron. 2003;40:881–884. doi: 10.1016/s0896-6273(03)00757-8. [DOI] [PubMed] [Google Scholar]
- 41.Lewis DA, Gonzalez-Burgos G. Nat Med. 2006;12:1016–1022. doi: 10.1038/nm1478. [DOI] [PubMed] [Google Scholar]
- 42.Spitzer RL, Williams JB, Gibbon M, First MB. User's Guide for the Structured Clinical Interview for DSM-III-R : SCID. Washington, DC: American Psychiatric Press; 1990. [Google Scholar]
- 43.Overall JE. In: Psychological Measurements in Psychopharmacology: Modern Problems in Pharmacopsychiatry. Pichot P, editor. Vol 7. Basel: Karger; 1974. pp. 267–301. [PubMed] [Google Scholar]
- 44.Andreasen NC. Scale for Assessment of Negative Symptoms: Technical Report. Iowa City: University of Iowa; 1983. [Google Scholar]
- 45.Andreasen NC. Scale for Assessment of Positive Symptoms: Technical Report. Iowa City: University of Iowa; 1983. [Google Scholar]
- 46.Spitzer RL, Williams JBW, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R: Non-Patient Edition. Washington, DC: American Psychiatric Press; 1990. Version 1.0. [Google Scholar]
- 47.Bertrand O, Perrin F, Pernier J. Electroencephalogr Clin Neurophysiol. 1985;62:462–464. doi: 10.1016/0168-5597(85)90058-9. [DOI] [PubMed] [Google Scholar]
- 48.Liddle PF. Br J Psychiatry. 1987;151:145–151. doi: 10.1192/bjp.151.2.145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.