Abstract
Macrophage pattern recognition receptors (PRRs) play key roles in innate immunity, but they also may contribute to disease processes under certain pathological conditions. We recently showed that engagement of the type A scavenger receptor (SRA), a PRR, triggers JNK-dependent apoptosis in endoplasmic reticulum (ER)-stressed macrophages. In advanced atherosclerotic lesions, the SRA, activated JNK, and ER stress are observed in macrophages, and macrophage death in advanced atheromata leads to plaque necrosis. Herein, we show that SRA ligands trigger apoptosis in ER-stressed macrophages by cooperating with another PRR, Toll-like receptor 4 (TLR4), to redirect TLR4 signaling from prosurvival to proapoptotic. Common SRA ligands activate both TLR4 signaling and engage the SRA. The TLR4 effect results in activation of the proapoptotic MyD88-JNK branch of TLR4, whereas the SRA effect silences the prosurvival IRF-3-IFN-β branch of TLR4. The normal cell-survival effect of LPS-induced TLR4 activation is converted into an apoptosis response by immunoneutralization of IFN-β, and the apoptosis effect of SRA ligands is converted into a cell-survival response by reconstitution with IFN-β. Thus, combinatorial signaling between two distinct PRRs results in a functional outcome-macrophage apoptosis that does not occur with either PRR alone. PRR-induced macrophage death may play important roles in advanced atherosclerosis and in other innate immunity-related processes in which the balance between macrophage survival and death is critical.
Keywords: apoptosis, atherosclerosis, scavenger receptor, Toll-like receptor, innate immunity
Macrophages play key roles in all stages of atherosclerosis (1). In advanced atheromata, macrophage apoptosis in the setting of defective phagocytic clearance leads to plaque necrosis (2). Plaque necrosis is thought to promote lesion instability, leading to myocardial infarction, stroke, and peripheral vascular disease (3–7). Thus, advanced lesional macrophage apoptosis may be a critical step in benign-to-vulnerable plaque transformation.
Based on mechanistic studies and known properties of advanced atherosclerotic lesions, we have proposed a “multihit” model of advanced lesional macrophage apoptosis. In this model, macrophage apoptosis is triggered by the endoplasmic reticulum (ER) stress pathway known as the unfolded protein response (UPR) in combination with engagement of the macrophage type A scavenger receptor (SRA), but not by either stimulus alone (8). The UPR is activated in advanced murine and human atherosclerotic lesions (9–11), and these lesions are known to contain a number of UPR activators (e.g., oxidant stress and nitric oxide) and SRA ligands (e.g., oxidized lipoproteins and advanced glycosylation end products) (12–14). Moreover, subpopulations of macrophages in apoptotic-rich areas of lesions are filled with lipoprotein-derived free cholesterol (FC) (15–19). In this scenario, the modified lipoproteins engage the SRA, and the lipoprotein-derived FC activates the UPR, leading to macrophage apoptosis (8). The UPR-SRA pathway of macrophage apoptosis requires activation of the protein kinase JNK (8), which also is known to be present in atheromata and promote atherogenesis (20). Despite the new insight gained from these studies, the mechanisms by which SRA engagement contributes to apoptosis and how JNK activation is linked to the UPR or SRA were not elucidated.
Because there is little evidence for direct signaling by the SRA (21), we reasoned that SRA may cooperate with another receptor to initiate apoptotic signaling. In particular, the SRA is a pattern recognition receptor (PRR) of the innate immune system (22), and recent work has demonstrated that different PRRs can functionally cooperate in macrophage–bacteria interactions and signaling (23, 24). In this context, we focused on a possible role for Toll-like receptors (TLRs) in cooperating with SRA, because TLRs are bona fide signaling PRRs (25, 26). Consistent with our hypothesis, we show here that the SRA alters TLR4 signaling in a manner that promotes macrophage apoptosis.
Results
TLR4 Is Required for SRA-Induced Apoptosis in ER-Stressed Macrophages.
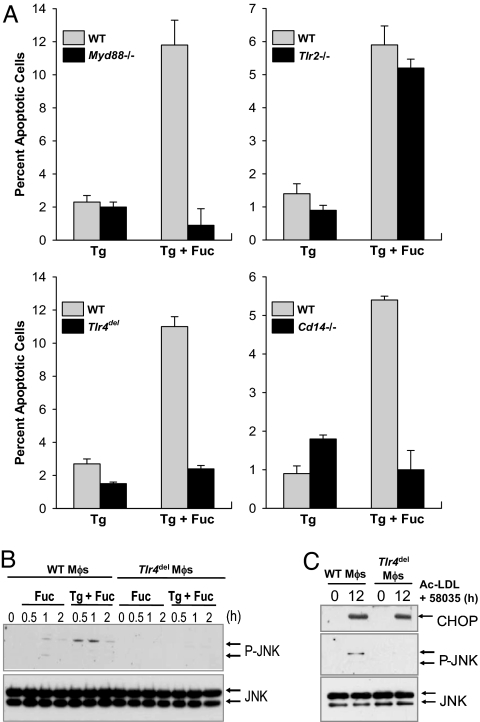
To determine whether TLR signaling in general was necessary for SRA-induced apoptosis in ER-stressed macrophages, we compared WT macrophages with those lacking the common TLR adaptor MyD88. We began with a simplified model of SRA-induced macrophage apoptosis (8), in which the SRA is engaged with the ligand fucoidan and the UPR is activated with the SERCA inhibitor thapsigargin. As shown in Fig. 1A, macrophages from Myd88−/− mice were resistant to apoptosis under these conditions, indicating an involvement of TLR signaling. Experiments with Tlr2−/− macrophages showed that this member of the TLR family was not involved in apoptosis. However, fucoidan/thapsigargin-induced apoptosis was markedly suppressed in macrophages lacking TLR4. Similar results were obtained by using FC loading (the SRA ligand acetyl-low-density lipoprotein (LDL) plus the cholesterol esterification inhibitor 58035) as the inducer of SRA-dependent apoptosis [see ref 8. and supporting information (SI) Fig. 5 A–C]. Of note, very prolonged FC loading causes necrosis of macrophages (27), which is independent of TLR4 (unpublished observations). TLR4 activation by LPS requires two additional components, CD14 and MD2 (25), and these molecules also were found to be essential for SRA/ER stress-induced macrophage apoptosis (Fig. 1A and SI Fig. 5D). Importantly, the critical requirement for TLR4 in macrophage apoptosis could not be explained by endotoxin contamination. All reagents used in these experiments contained <0.1 endotoxin units/ml endotoxin as determined by the Limulus Amebocyte Lysate (LAL) assay. Moreover, neither apoptosis nor the TLR4-signaling reactions described below could be replicated by incubating macrophages with an amount of endotoxin that was 2- to 5-fold greater than the minute amounts detected in the LAL assay. Finally, as will become evident in the following data, TLR4 signaling triggered by acetyl-LDL or fucoidan was found to be fundamentally different from that induced by higher amounts of endotoxin.
Fig. 1.
The TLR4 pathway is necessary for SRA-dependent apoptosis of ER-stressed macrophages. (A) Macrophages from WT, Myd88−/−, Tlr2−/−, Tlr4del, or Cd14−/− mice were incubated for 24 h with 0.5 μM thapsigargin (Tg) or thapsigargin plus 50 μg/ml fucoidan (Fuc). The cells then were stained with Alexa 488 annexin-V and propidium iodide and quantified for apoptosis. (B and C) WT or Tlr4del macrophages were incubated for the indicated times with fucoidan, thapsigargin plus fucoidan, or 100 μg/ml acetyl-LDL (Ac-LDL) plus 10 μg/ml compound 58035. Cell lysates then were immunoblotted for Thr-183/Thr-185-phospho-JNK (P-JNK), total JNK, and CHOP.
The scavenger receptor family contains another member, CD36, which can bind certain SRA ligands and, in addition, functionally interact with TLRs (23, 24). We therefore tested whether CD36 was necessary for fucoidan-induced apoptosis in ER-stressed macrophages. Whereas fucoidan-induced apoptosis in ER-stressed macrophages was suppressed markedly in Sra−/− macrophages (8), fucoidan-induced apoptosis was similar in WT vs. Cd36−/− ER-stressed macrophages (data not shown).
SRA Ligands, Acting Through TLR4 but Not SRA, Activate NF-κB and JNK in ER-Stressed Macrophages.
The finding that TLR4 is necessary for SRA-induced apoptosis in ER-stressed macrophages raised the question as to whether SRA ligands activate TLR4 signaling, a scenario consistent with previous studies using atherogenic lipoproteins that are SRA ligands (28). If so, the model would involve two distinct actions of SRA ligands, activation of TLR4 and engagement of the SRA itself, that somehow combine to induce apoptosis in ER-stressed macrophages. To begin, we focused on the MyD88-Mal branch of TLR4 signaling, which results in nuclear translocation of NF-κΒ-p65 and activation of MAPKs such as JNK (26). We first showed that endotoxin-free fucoidan plus thapsigargin activated IRAK1 (data not shown), which functions immediately downstream of MyD88. We then tested whether SRA engagement activated the NF-κB pathway in ER-stressed macrophages in a TLR4-dependent manner. As shown in SI Fig. 6, treatment of macrophages with endotoxin-free fucoidan plus thapsigargin led to both degradation of I-κBα and nuclear translocation of p65, two key steps in NF-κB activation. Similar results were found by using FC-enriched macrophages (29). Functional significance of SRA ligand-induced NF-κB activation was demonstrated by the finding that the p65-responsive genes TNFα, IL-6, and iNOS were induced by fucoidan plus thapsigargin (SI Fig. 6B, gray bars, and SI Fig. 6C, WT Mφs). Most relevant to macrophage apoptosis, fucoidan plus thapsigargin as well as FC loading with the SRA ligand acetyl-LDL activated proapoptotic JNK (Fig. 1 B and C, WT Mφs). Importantly, the expression of p65-inducible genes and activation of JNK by fucoidan and acetyl-LDL were dependent on both TLR4 and MD2 (SI Fig. 6B, black bars; SI Fig. 6C and Fig. 1 B and C, Tlr4delMφs; and SI Fig. 7).
Given that TLR4 is critical for the activation of NF-κB and proapoptotic JNK by endotoxin-free SRA ligands, does engagement of the SRA itself by these ligands play a role in these two processes? Significantly, we found that SRA ligands induced iNOS and activated JNK equally well in WT vs. Sra−/− ER-stressed macrophages (data not shown), indicating that the SRA was not involved in NF-κB gene expression and JNK activation. We then considered a role for the SRA in another key component of our multihit model, namely induction of the proapoptotic UPR effector CHOP (GADD153) (9), because this pathway does not involve TLR4 (Fig. 1C Top). However, our previous studies showed that induction of CHOP in our model was also independent of the SRA (8). Together, these results point to the existence of two complementary but distinct proapoptotic pathways, both of which are SRA-independent: TLR4-dependent JNK activation and TLR4-independent CHOP expression. The fact that neither pathway requires SRA (above) but that SRA is necessary for macrophage apoptosis (8) raised the intriguing question as to how SRA functions as a critical component in death signaling. This question is addressed after the following section.
Cytoplasmic Calcium Is Necessary for TLR4-JNK Activation and Apoptosis in ER-Stressed Macrophages.
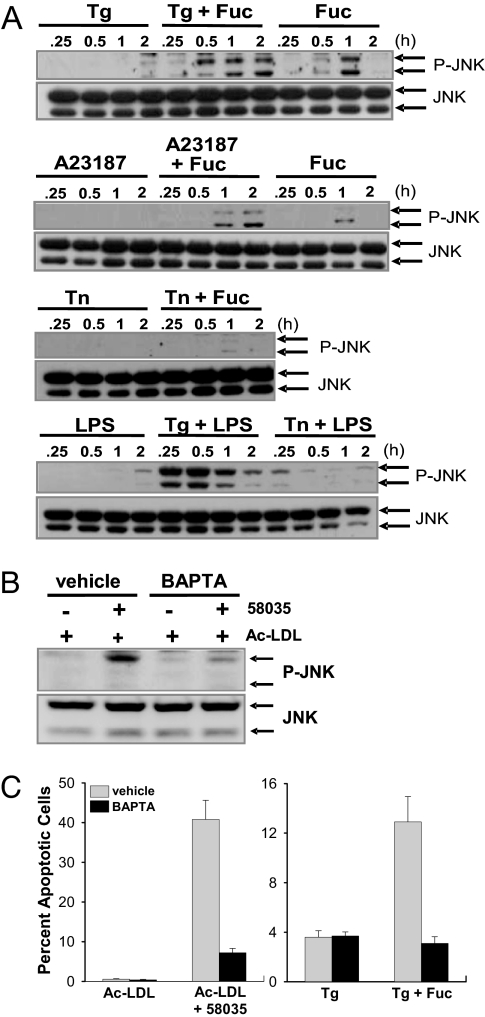
The above data show that fucoidan without thapsigargin is only a very weak activator of TLR4-dependent cytokine induction or JNK activation. Similar data were obtained with acetyl-LDL alone (29). Thus, fucoidan and acetyl-LDL activate TLR4-MyD88 signaling only under conditions of thapsigargin treatment or FC enrichment, respectively. Based on our previous studies, we knew that at least one role of these treatments is their ability to activate the CHOP branch of the UPR (9, 30). However, these treatments, like many other UPR activators, also block calcium reuptake from the cytoplasm into the ER lumen (9, 30). Therefore, we sought to determine whether perturbations of cellular calcium also might be important. Indeed, previous work has suggested a role for cytoplasmic calcium in the TLR4 pathway (31). To investigate this point, we directly compared fucoidan-induced JNK activation in macrophages treated with three different UPR activators: thapsigargin; the calcium ionophore A23187; and the protein glycosylation inhibitor tunicamycin, which does not affect cellular calcium. As shown in the top three blots in Fig. 2A, JNK activation in fucoidan-treated macrophages was enhanced and sustained by both thapsigargin and A23187, whereas tunicamycin had no such effect. Similar results were found in comparing the effects of thapsigargin and tunicamycin on LPS-induced JNK activation (Fig. 2A Bottom). Consistent with the JNK data, tunicamycin plus fucoidan did not induce macrophage apoptosis (data not shown).
Fig. 2.
Cytoplasmic Ca2+ mobilization is necessary for enhanced TLR4-dependent JNK activation and apoptosis. (A) Macrophages were incubated for 0.25–2 h with the indicated reagents, alone or in combination: thapsigargin (Tg; 0.5 μM), fucoidan (Fuc; 50 μg/ml), A23187 (2 μg/ml), tunicamycin (Tn; 2 μg/ml), and LPS (500 pg/ml). Cell lysates were immunoblotted for Thr-183/Thr-185-phospho-JNK (P-JNK) and total JNK. (B) Macrophages were preincubated for 10 min with vehicle control or 15 μM BAPTA. The cells then were treated with 50 μg/ml acetyl-LDL (Ac-LDL) or acetyl-LDL plus 58035 (FC loading) for 12 h. Lysates were immunoblotted for Thr-183/Thr-185-phospho-JNK, and total JNK. (C) Macrophages were preincubated for 10 min with vehicle control or 5 μM BAPTA-AM. The cells then were incubated for 24 h with 50 μg/ml acetyl-LDL alone or in combination with 58035, 0.5 μM thapsigargin, or thapsigargin plus 50 μg/ml fucoidan. The cells were assayed for apoptosis.
These data raised the possibility that cytoplasmic calcium played an important role in fucoidan-induced JNK activation and apoptosis. We therefore determined whether chelating cytoplasmic Ca2+ with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (BAPTA)-AM could prevent TLR4-induced JNK activation and apoptosis. As shown in Fig. 2B, pretreatment of macrophages with BAPTA-AM markedly suppressed JNK activation in FC-enriched macrophages. Most importantly, apoptosis induced by both FC enrichment and thapsigargin plus fucoidan was markedly inhibited by BAPTA-AM (Fig. 2C). Thus, the subset of UPR activators that function by perturbing cellular calcium homeostasis play roles in two pathways critical for macrophage apoptosis: They induce proapoptotic CHOP, and they increase cytoplasmic calcium, which is necessary for maximal induction of the TLR4-JNK proapoptotic pathway.
Fucoidan and Acetyl-LDL Promote Apoptosis in ER-Stressed Macrophages by Suppressing the IRF3-IFN-β Cell-Survival Branch of TLR4 Signaling.
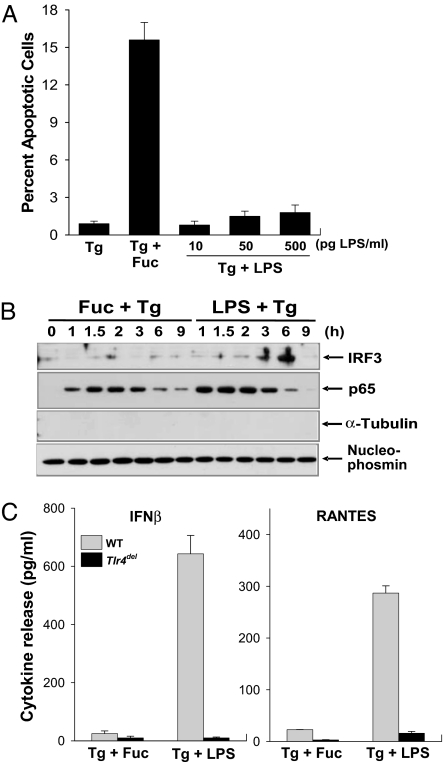
The section before last suggested a model in which the SRA played a critical role in macrophage apoptosis independently of the TLR4-JNK pathway. If so, a TLR4 activator that did not activate this putative proapoptotic SRA pathway should not trigger apoptosis in ER-stressed macrophages. To test this prediction, we determined whether the TLR4 activator LPS could induce apoptosis in ER-stressed macrophages in the absence of an SRA ligand. As shown in Fig. 3A, 500 pg/ml LPS was unable to trigger apoptosis in control or ER-stressed macrophages, whereas there was increased apoptosis with fucoidan in ER-stressed macrophages. As expected from our earlier data above, fucoidan and LPS (500 pg/ml) were equally effective in stimulating the TLR4-MyD88 pathway in ER-stressed macrophages, as indicated by assays of TNFα and iNOS (data not shown). Thus, even though SRA ligands and LPS stimulate the TLR4-MyD88 pathway to a similar extent, their effects on the survival-death balance in macrophages are very different. These data further highlight the critical role for the SRA in apoptosis of ER-stressed macrophages.
Fig. 3.
Fucoidan-induced suppression of the IRF3-IFN-β branch of TLR4 signaling correlates with apoptosis in ER-stressed macrophages. (A) Macrophages were incubated for 24 h with thapsigargin (Tg), thapsigargin plus fucoidan (Fuc), or thapsigargin plus increasing concentrations of LPS. The cells then were assayed for apoptosis. (B) Macrophages were incubated for the indicated times with thapsigargin plus fucoidan or with thapsigargin plus 500 ng/ml LPS. Nuclear extracts were immunoblotted for IRF3, p65, α-tubulin, and nucleophosmin. (C) WT or Tlr4del macrophages were incubated for 6 h with thapsigargin plus fucoidan or with thapsigargin plus LPS. The media were collected and assayed by ELISA for IFN-β and RANTES.
In addition to activating the MyD88-Mal branch, LPS also activates a TLR4 branch involving the adaptor pair TRIF-TRAM (32). Significantly, the TRIF-TRAM branch has cell-survival potential through the activation of the IRF3-IFN-β pathway (33–35). In this context, we hypothesized that SRA engagement might somehow suppress this branch of the TLR4 pathway. In support of this hypothesis, we found that the SRA ligand fucoidan was a relatively poor inducer of nuclear IRF3 in ER-stressed macrophages compared with LPS (Fig. 3B Top). Consistent with our previous data on the MyD88 pathway, fucoidan and LPS had similar effects on promoting p65 nuclear translocation (Fig. 3B Middle Top). Moreover, TLR4-dependent expression of two IRF3-inducible genes, IFN-β and RANTES, was much less in macrophages treated with fucoidan plus thapsigargin compared with macrophages exposed to LPS plus thapsigargin (Fig. 3C). Significantly, fucoidan blocked nuclear IRF3 even in the presence of LPS, demonstrating a dominant effect of the SRA ligand as an IRF3 suppressor (SI Fig. 8A).
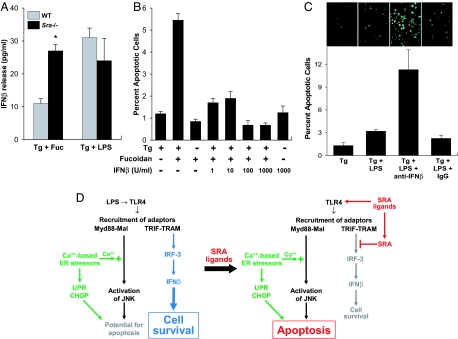
To determine whether fucoidan was acting through SRA to suppress IFN-β, we compared IFN-β production in WT vs. Sra−/− ER-stressed macrophages. As shown by Fig. 4A Left, fucoidan-induced IFN-β production was substantially greater in Sra−/− macrophages, consistent with an IFN-β-suppressive role of the SRA. In contrast, SRA deficiency did not influence LPS-induced IFN-β production (Fig. 4A Right). Enhancement of IFN-β also was observed when SRA was blocked by using an anti-SRA antibody and when acetyl-LDL was used as the SRA ligand instead of fucoidan (SI Fig. 8B). Consistent with these data, the IRF3-dependent cytokine RANTES also was enhanced by SRA inhibition (SI Fig. 8C). These data and those above suggest that SRA ligands have two independent effects in ER-stressed macrophages: In a TLR4-depedendent manner, they activate or induce molecules downstream of MyD88-Mal (i.e., IRAK1, TNFα, IL-6, and JNK), and in an SRA-dependent manner, they suppress molecules downstream of TRIF-TRAM (i.e., IRF3, IFN-β, and RANTES).
Fig. 4.
SRA-dependent suppression of IFN-β contributes to macrophage apoptosis. (A) WT or Sra−/− macrophages were incubated for 6 h with thapsigargin plus fucoidan (Tg + Fuc) or thapsigargin plus 500 pg/ml LPS. The media then were assayed for IFN-β (∗, P < 0.001 vs. WT Tg + Fuc). (B) Macrophages were incubated for 18 h with thapsigargin alone, fucoidan alone, thapsigargin/fucoidan plus increasing concentrations of recombinant IFN-β, or IFN-β alone. The cells then were assayed for apoptosis as described in Fig. 1. (C) Macrophages were incubated for 24 h with thapsigargin, thapsigargin plus 1 ng/ml LPS, thapsigargin/LPS plus 50 units/ml anti-IFN-β neutralizing antibody, or thapsigargin/LPS plus an equivalent amount of rabbit IgG control antibody. The cells then were assayed for apoptosis. Representative fluorescent images and quantitative apoptosis data for each condition are shown. (D) Model of SRA-mediated alteration of TLR4 signaling from prosurvival to proapoptosis. See text for details.
To investigate the possible proapoptotic role of IFN-β suppression in SRA ligand-induced apoptosis, we determined whether adding back this cytokine to SRA-engaged macrophages could block apoptosis. As shown in Fig. 4B, the addition of as little as 1 unit (≈40 pg) of IFN-β protected ER-stressed macrophages from apoptosis by fucoidan. We next addressed the converse issue, i.e., whether inhibiting IFN-β could convert the LPS survival response into an apoptosis response. As shown in Fig. 4C, a neutralizing antibody against IFN-β significantly increased apoptosis in ER-stressed macrophages treated with LPS.
Based on these data and those above, we propose that in macrophages exposed to LPS, activation of a “dominant” TRIF-TRAM-IRF3-IFN-β pathway promotes cell survival even though a potential proapoptotic response is activated via the MyD88-JNK pathway (Fig. 4D Left). However, SRA ligands selectively activate the proapoptotic MyD88-JNK branch through TLR4 while muting the prosurvival IRF3-IFN-β branch through SRA (Fig. 4D Right). When combined with ER stress-induced CHOP expression and calcium mobilization, this multihit pathway induces macrophage apoptosis.
Discussion
Our study provides evidence of how combinatorial PRR signaling between TLR4 and SRA in ER-stressed macrophages converts TLR4 signaling into an apoptosis pathway. Although the original pathophysiologic context of this work was based on the importance of macrophage death in advanced atherosclerosis, it is possible that our findings may have broader implications to innate immunity. For example, macrophage death induced by combinatorial PRR signaling may be a host defense mechanism against those pathogenic organisms that seek safe haven inside living macrophages (36–38). With regard to evidence that SRA and TLR4 may functionally interact in vivo, three independent reports have shown that mice lacking SRA have altered cytokine and survival responses to LPS (39–41), although the mechanisms of the altered responses were not elucidated.
In terms of atherosclerosis, lesional macrophages express both TLR4 and SRA (14, 22, 42, 43), and lesions contain a number of SRA ligands, including atherogenic lipoproteins, advanced glycosylation end products, and amyloid-β (12, 14, 44). Moreover, exactly as predicted from our model, a microarray study listed IRF-3 among those genes suppressed in human macrophages exposed to oxidized LDL (45), an SRA ligand that exists in atherosclerotic lesions. Although previous studies have reported the effects of SRA and TLR4 pathway deficiencies on murine atherosclerosis, in some cases with contradictory results, all of these studies used early to mid-stage lesion area as the endpoint (14, 42, 46, 47). The model described here is specifically relevant to processes involved in advanced lesion morphology, and so the roles of SRA and TLR4 on advanced lesional macrophage death and plaque necrosis represent an important area for future study. Advanced plaques also contain molecules or conditions that promote calcium-based ER stress, including atherogenic lipoproteins and peroxynitrite (8, 13), and lesional macrophages display multiple markers of UPR activation in vivo (9, 10). Moreover, we recently found that the apoptosis pathway described here is enhanced in insulin-resistant macrophages both in vitro and in advanced atherosclerotic lesions, and the advanced lesions of humans with type II diabetes are characterized by increased lesional macrophage death and plaque necrosis (48, 49). Thus, PRR-mediated macrophage apoptosis in advanced atherosclerotic lesions may be relevant particularly in the setting of insulin resistance.
At a cell biological level, the findings reported here raise a number of mechanistic questions related to the mechanism by which SRA engagement suppresses IRF-3-IFN-β, the step in the TLR4-IFN-β pathway that is suppressed by SRA engagement, the downstream prosurvival molecules induced by IFN-β, and the mechanism by which perturbation of cellular calcium metabolism enhances the TLR4-MyD88 pathway. Moreover, IRF3 also can function as a coactivator of NF-κB-regulated genes (50), and so inhibition of IRF3 by SRA might prevent activation of genes involved in the inflammatory response. This hypothesis may provide insight into how SRA affects the inflammatory response to LPS in vivo (39–41). Addressing these questions and probing combinatorial PRR apoptosis signaling in vivo may yield new therapeutic strategies related to diseases and host defense processes in which the balance between macrophage survival and death plays important roles.
Materials and Methods
The materials and methods used for preparation of lipoproteins, endotoxin testing, immunoblot analysis, cytokine ELISA, and statistics are in SI Materials and Methods.
Mice.
The following mice were purchased from The Jackson Laboratory (Bar Harbor, ME): C57BL/6 mice, Tlr2−/− mice (strain B6.129 Tlr2tm1Kir/J) backcrossed to the C57BL/6J background, Tlr4del mice (strain C57BL/10ScNJ), Tlr4control mice (strain C57BL/10ScSnJ), and Cd14−/− mice (strain B6.129S-Cd14tm1Frm/J) backcrossed to the C57BL/6J background. Myd88−/−, Sra−/− (Msr−/−), Cd36−/−, and Md2−/− mice were described in refs. 8, 24, 51, and 52. Macrophages from female 8- to 10-week C57BL/6J mice were used as WT controls in experiments in which the gene-targeted mice were on the C57BL/6J background, i.e., all mice except Tlr4del. For the experiments using Tlr4del mice, the WT controls are indicated above.
Mouse Peritoneal Macrophages.
Peritoneal macrophages from adult female C57BL/6J mice and all mutant mice used in this study were harvested 3 days after i.p. injection of Con A or 4 days after i.p. injection of methyl-BSA in mice previously immunized with this antigen (29). Macrophages were harvested 4 days later by peritoneal lavage and maintained in medium containing DMEM, 10% FBS, and 20% l-cell-conditioned medium. The medium was replaced every 24 h until cells reached 90% confluency. For the BAPTA experiments, macrophages were pretreated for 10 min with 5 or 15 μM BAPTA-AM plus 0.02% F127 Pleuronic; 0.02% F127 Pleuronic in DMSO served as the vehicle control.
Cell Death Assays.
After treatment, macrophages were assayed for early to mid-stage apoptosis by staining with Alexa 488-conjugated Annexin V (green) and late-stage apoptosis by costaining with propidium iodide (red), as described in ref. 8. Cells were viewed immediately at room temperature by using an Olympus IX-70 inverted fluorescent microscope equipped with a mercury 100W lamp (CHIU Technical Corp., Kings Park, NY), filter wheels, fluorescent filters (Chroma, Brattleboro, VT), an Olympus LCPlanF1 ×20 objective, Imaging software (Roper Scientific, Tucson, AZ), and a Cool Snap CCD camera and (RS Photometrics, Tucson, AZ). Representative fields (for to six fields containing ≈1,000 cells) were photographed for each condition. The number of Annexin V- and PI-positive were counted and expressed as a percent of the total number of cells in at least four separate fields from duplicate wells.
Acknowledgments
We thank Drs. Mason Freeman (Massachusetts General Hospital) and Wahseng Lim (Columbia University) for helpful discussions; Drs. Anthony Ferrante and Carl deLuca (Columbia University) for providing the MyD88-deficient mice; and Liping Bao, Yuan Zhang, and Michelle Rappaport for technical assistance. This work was supported by National Institute of Health Grants HL79801 (to T.A.S.), GM54060 (D.T.G.), and HL75662 and HL57560 (to I.T.).
Abbreviations
- BAPTA
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate
- ER
endoplasmic reticulum
- FC
free cholesterol
- LDL
low-density lipoprotein
- PRR
pattern recognition receptor
- SRA
type A scavenger receptor
- TLR
Toll-like receptor
- UPR
unfolded protein response.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0609671104/DC1.
References
- 1.Glass CK, Witztum JL. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 2.Tabas I. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 3.Ball RY, Stowers EC, Burton JH, Cary NR, Skepper JN, Mitchinson MJ. Atherosclerosis. 1995;114:45–54. doi: 10.1016/0021-9150(94)05463-s. [DOI] [PubMed] [Google Scholar]
- 4.Kolodgie FD, Narula J, Burke AP, Haider N, Farb A, Hui-Liang Y, Smialek J, Virmani R. Am J Pathol. 2000;157:1259–1268. doi: 10.1016/S0002-9440(10)64641-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Libby P, Geng YJ, Aikawa M, Schoenbeck U, Mach F, Clinton SK, Sukhova GK, Lee RT. Curr Opin Lipidol. 1996;7:330–335. doi: 10.1097/00041433-199610000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Mitchinson MJ, Hardwick SJ, Bennett MR. Curr Opin Lipidol. 1996;7:324–329. doi: 10.1097/00041433-199610000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Ross R. Annu Rev Physiol. 1995;57:791–804. doi: 10.1146/annurev.ph.57.030195.004043. [DOI] [PubMed] [Google Scholar]
- 8.DeVries-Seimon T, Li Y, Yao PM, Stone E, Wang Y, Davis RJ, Flavell R, Tabas I. J Cell Biol. 2005;171:61–73. doi: 10.1083/jcb.200502078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, et al. Nat Cell Biol. 2003;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 10.Zhou J, Lhotak S, Hilditch BA, Austin RC. Circulation. 2005;111:1814–1821. doi: 10.1161/01.CIR.0000160864.31351.C1. [DOI] [PubMed] [Google Scholar]
- 11.Gargalovic PS, Gharavi NM, Clark MJ, Pagnon J, Yang WP, He A, Truong A, Baruch-Oren T, Berliner JA, Kirchgessner TG, et al. Arterioscler Thromb Vasc Biol. 2006;26:2490–2496. doi: 10.1161/01.ATV.0000242903.41158.a1. [DOI] [PubMed] [Google Scholar]
- 12.Baynes JW, Thorpe SR. Free Radic Biol Med. 2000;28:1708–1716. doi: 10.1016/s0891-5849(00)00228-8. [DOI] [PubMed] [Google Scholar]
- 13.Dickhout JG, Hossain GS, Pozza LM, Zhou J, Lhotak S, Austin RC. Arterioscler Thromb Vasc Biol. 2005;25:2623–2629. doi: 10.1161/01.ATV.0000189159.96900.d9. [DOI] [PubMed] [Google Scholar]
- 14.Platt N, Gordon S. J Clin Invest. 2001;108:649–654. doi: 10.1172/JCI13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burke AP, Virmani R, Galis Z, Haudenschild CC, Muller JE. J Am Coll Cardiol. 2003;41:1874–1886. doi: 10.1016/s0735-1097(03)00359-0. [DOI] [PubMed] [Google Scholar]
- 16.Katz SS, Shipley GG, Small DM. J Clin Invest. 1976;58:200–211. doi: 10.1172/JCI108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kruth HS, Fry DL. Exp Mol Pathol. 1984;40:288–294. doi: 10.1016/0014-4800(84)90046-7. [DOI] [PubMed] [Google Scholar]
- 18.Rapp JH, Connor WE, Lin DS, Inahara T, Porter JM. J Lipid Res. 1983;24:1329–1335. [PubMed] [Google Scholar]
- 19.Shio H, Haley NJ, Fowler S. Lab Invest. 1979;41:160–167. [PubMed] [Google Scholar]
- 20.Ricci R, Sumara G, Sumara I, Rozenberg I, Kurrer M, Akhmedov A, Hersberger M, Eriksson U, Eberli FR, Becher B, et al. Science. 2004;306:1558–1561. doi: 10.1126/science.1101909. [DOI] [PubMed] [Google Scholar]
- 21.Kim WS, Ordija CM, Freeman MW. Biochem Biophys Res Commun. 2003;310:542–549. doi: 10.1016/j.bbrc.2003.09.049. [DOI] [PubMed] [Google Scholar]
- 22.Greaves DR, Gordon S. J Lipid Res. 2005;46:11–20. doi: 10.1194/jlr.R400011-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zahringer U, et al. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 24.Stuart LM, Deng J, Silver JM, Takahashi K, Tseng AA, Hennessy EJ, Ezekowitz RA, Moore KJ. J Cell Biol. 2005;170:477–485. doi: 10.1083/jcb.200501113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fitzgerald KA, Rowe DC, Golenbock DT. Microbes Infect. 2004;6:1361–1367. doi: 10.1016/j.micinf.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Takeda K, Akira S. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Warner GJ, Stoudt G, Bamberger M, Johnson WJ, Rothblat GH. J Biol Chem. 1995;270:5772–5778. doi: 10.1074/jbc.270.11.5772. [DOI] [PubMed] [Google Scholar]
- 28.Miller YI, Viriyakosol S, Worrall DS, Boullier A, Butler S, Witztum JL. Arterioscler Thromb Vasc Biol. 2005;25:1213–1219. doi: 10.1161/01.ATV.0000159891.73193.31. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Schwabe RF, DeVries-Seimon T, Yao PM, Gerbod-Giannone MC, Tall AR, Davis RJ, Flavell R, Brenner DA, Tabas I. J Biol Chem. 2005;280:21763–21772. doi: 10.1074/jbc.M501759200. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Ge M, Ciani L, Kuriakose G, Westover E, Dura M, Covey D, Freed JH, Maxfield FR, Lytton J, et al. J Biol Chem. 2004;279:37030–37039. doi: 10.1074/jbc.M405195200. [DOI] [PubMed] [Google Scholar]
- 31.Chen BC, Hsieh SL, Lin WW. J Leukoc Biol. 2001;69:280–288. [PubMed] [Google Scholar]
- 32.Rowe DC, McGettrick AF, Latz E, Monks BG, Gay NJ, Yamamoto M, Akira S, O'Neill LA, Fitzgerald KA, Golenbock DT. Proc Natl Acad Sci USA. 2006;103:6299–6304. doi: 10.1073/pnas.0510041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang Z, Georgel P, Du X, Shamel L, Sovath S, Mudd S, Huber M, Kalis C, Keck S, Galanos C, et al. Nat Immunol. 2005;6:565–570. doi: 10.1038/ni1207. [DOI] [PubMed] [Google Scholar]
- 34.Scheel-Toellner D, Pilling D, Akbar AN, Hardie D, Lombardi G, Salmon M, Lord JM. Eur J Immunol. 1999;29:2603–2612. doi: 10.1002/(SICI)1521-4141(199908)29:08<2603::AID-IMMU2603>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 35.Wang K, Scheel-Toellner D, Wong SH, Craddock R, Caamano J, Akbar AN, Salmon M, Lord JM. J Immunol. 2003;171:1035–1041. doi: 10.4049/jimmunol.171.2.1035. [DOI] [PubMed] [Google Scholar]
- 36.Ernst JD. Infect Immun. 1998;66:1277–1281. doi: 10.1128/iai.66.4.1277-1281.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peiser L, Gough PJ, Kodama T, Gordon S. Infect Immun. 2000;68:1953–1963. doi: 10.1128/iai.68.4.1953-1963.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peiser L, de Winther MP, Makepeace K, Hollinshead M, Coull P, Plested J, Kodama T, Moxon ER, Gordon S. Infect Immun. 2002;70:5346–5354. doi: 10.1128/IAI.70.10.5346-5354.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fulton WB, Reeves RH, Takeya M, De Maio A. J Immunol. 2006;176:3767–3773. doi: 10.4049/jimmunol.176.6.3767. [DOI] [PubMed] [Google Scholar]
- 40.Haworth R, Platt N, Keshav S, Hughes D, Darley E, Suzuki H, Kurihara Y, Kodama T, Gordon S. J Exp Med. 1997;186:1431–1439. doi: 10.1084/jem.186.9.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kobayashi Y, Miyaji C, Watanabe H, Umezu H, Hasegawa G, Abo T, Arakawa M, Kamata N, Suzuki H, Kodama T, et al. J Pathol. 2000;192:263–272. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH692>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 42.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Proc Natl Acad Sci USA. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michelsen KS, Doherty TM, Shah PK, Arditi M. J Immunol. 2004;173:5901–5907. doi: 10.4049/jimmunol.173.10.5901. [DOI] [PubMed] [Google Scholar]
- 44.De Meyer GR, De Cleen DM, Cooper S, Knaapen MW, Jans DM, Martinet W, Herman AG, Bult H, Kockx MM. Circ Res. 2002;90:1197–1204. doi: 10.1161/01.res.0000020017.84398.61. [DOI] [PubMed] [Google Scholar]
- 45.Marson A, Lawn RM, Mikita T. J Biol Chem. 2004;279:28781–28788. doi: 10.1074/jbc.M313207200. [DOI] [PubMed] [Google Scholar]
- 46.Bjorkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT, et al. Nat Med. 2004;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 47.Moore KJ, Kunjathoor VV, Koehn SL, Manning JJ, Tseng AA, Silver JM, McKee M, Freeman MW. J Clin Invest. 2005;115:2192–2201. doi: 10.1172/JCI24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han S, Liang CP, DeVries-Seimon T, Ranalletta M, Welch CL, Collins-Fletcher K, Accili D, Tabas I, Tall AR. Cell Metab. 2006;3:257–266. doi: 10.1016/j.cmet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 49.Virmani R, Burke AP, Kolodgie F. Can J Cardiol. 2006;22(Suppl B):81B–84B. doi: 10.1016/s0828-282x(06)70991-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ogawa S, Lozach J, Benner C, Pascual G, Tangirala RK, Westin S, Hoffmann A, Subramaniam S, David M, Rosenfeld MG, et al. Cell. 2005;122:707–721. doi: 10.1016/j.cell.2005.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 52.Nagai Y, Akashi S, Nagafuku M, Ogata M, Iwakura Y, Akira S, Kitamura T, Kosugi A, Kimoto M, Miyake K. Nat Immunol. 2002;3:667–672. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]