Abstract
It is a common experience to sacrifice sleep to meet the demands of our 24-h society. Current estimates reveal that as a society, we sleep on average 2 h less than we did 40 years ago. This level of sleep restriction results in negative health outcomes and is sufficient to produce cognitive deficits and reduced attention and is associated with increased risk for traffic and occupational accidents. Unfortunately, there is no simple quantifiable marker that can detect an individual who is excessively sleepy before adverse outcomes become evident. To address this issue, we have developed a simple and effective strategy for identifying biomarkers of sleepiness by using genetic and pharmacological tools that dissociate sleep drive from wake time in the model organism Drosophila melanogaster. These studies have identified a biomarker, Amylase, that is highly correlated with sleep drive. More importantly, both salivary Amylase activity and mRNA levels are also responsive to extended waking in humans. These data indicate that the fly is relevant for human sleep research and represents a first step in developing an effective method for detecting sleepiness in vulnerable populations.
Keywords: Drosophila, saliva, sleep deprivation
It has been suggested that forced and self-inflicted sleep loss have reached epidemic proportions in Western industrialized populations (1, 2), costing billions of dollars in lost productivity and creating hazardous conditions on our roadways (3), in our skies (4), and in our hospitals (5). The National Highway Traffic Safety Administration estimates that 20% of motor vehicle crashes are attributed to sleepiness and fully 37% of adult drivers report falling asleep at the wheel at some point in their lives. Moreover, both regional and long-haul pilots accumulate sleep debt during trips, fall asleep in the cockpit, and experience levels of sleepiness that are associated with performance decrements (6, 7). In the hospital setting, training demands frequently disrupt the sleep of medical residents, which is then associated with increased attentional failures and medical errors (8). Indeed, after a heavy call rotation, the driving performance of medical residents was similar to those with a blood alcohol level of 0.05 g % (9) and is associated with increased risk of falling asleep while driving (10).
Given the magnitude of this problem, it is not surprising that the sleep community, public health officials, and others have devoted considerable attention toward minimizing the negative impact of sleep loss on public health and safety (11). In addition to more focused basic research and increased educational campaigns to create public awareness, both regulatory and legislative initiatives have been implemented to address this problem. A general theme that has emerged from all of these efforts has been the importance of identifying a simple and quantifiable biomarker of sleepiness (11–13). A biomarker of sleepiness should be responsive to increasing levels of sleep debt and should only be activated by periods of waking that are followed by compensatory increases in sleep time (sleep homeostasis). Unfortunately, identifying such a marker in humans poses significant challenges.
In this work, we outline a strategy for identifying biomarkers of sleepiness using the model organism Drosophila melanogaster. This strategy is based on a unique set of genetic, behavioral, and pharmacological tools that have been identified in the fly that allow for the disassociation of wake time with sleep drive (14–16). By using this approach, we have identified a molecule, Amylase, which is highly correlated with sleep drive in the fly and is also responsive to waking in humans.
Results
Saliva is a suitable tissue for identifying biomarkers of sleepiness in humans, given that brainstem nuclei regulating salivary gland activity receive inputs from neural structures in the forebrain that are centers for homeostatic regulation (17, 18). In addition, saliva contains ≈3,000 mRNA species (19). We wondered whether any of these genes could be used as biomarkers of sleepiness. To reduce the number of candidate genes that need to be tested in humans, we first looked for likely candidates by using results obtained from several independent Drosophila microarray studies. Amylase was consistently modified by sleep loss using a variety of sleep deprivation (SD) protocols. This observation coupled with the fact that amylase is abundant in a readily accessible fluid in humans prompted the current set of experiments.
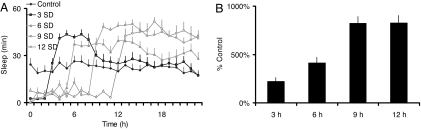
To be effective, a biomarker should be responsive to increasing levels of sleep debt and should only be activated by periods of waking that are followed by compensatory increases in sleep time (sleep homeostasis). With this in mind, we have evaluated the reliability of Amylase as a biomarker of sleep drive by using genetic and pharmacological tools that differentially activate homeostatic mechanisms in the model organism D. melanogaster. First, we evaluated the temporal dynamics of Amylase mRNA in flies mutant for the canonical clock gene cycle (cyc01) after 3, 6, 9, and 12 h of SD. The detrimental effects of waking are accelerated in cyc01 mutants and accrue over the course of a short and well defined interval measured in hours (15, 20). As seen in Fig. 1A, even small amounts of sleep loss (3 h) result in large compensatory increases in sleep. Importantly, as cyc01 flies experience greater amounts of sleep loss, they become increasingly longer sleepers. Amylase mRNA levels increased progressively with the duration of waking indicating that it is responsive to increasing levels of sleep debt (Fig. 1B).
Fig. 1.
Amylase is responsive to increasing levels of sleep debt. (A) With increasing amounts of sleep loss, cyc01 flies become longer sleepers. (B) Relative expression of Amylase mRNA extracted from whole heads is progressively increased after 3, 6, 9, and 12 h of SD in the sleep-loss sensitive mutant cyc01 as assessed by quantitative PCR (n = 24 per condition).
Next, we evaluated whether Amylase expression is associated with conditions where sleep drive is high or whether it is nonspecifically activated by waking. To accomplish this goal, we evaluated the temporal dynamics of Amylase expression in flies mutant for timeless (tim01) after 3, 6, 9, and 12 h of SD (Fig. 2A). tim01 flies are specifically resistant to short-term SD (3 and 6 h) but exhibit a normal homeostatic response after 9 and 12 h of SD (14, 15). As seen in Fig. 2B, Amylase mRNA levels were elevated after SD durations that activate sleep homeostatic mechanisms (9 and 12 h SD; filled bars). To determine whether these results could be generalized to other experimental conditions, we induced periods of waking that differentially activated sleep homeostasis by using caffeine and methamphetamine. Both caffeine and methamphetamine each produce sustained periods of waking and similar locomotor activity profiles. However, unlike caffeine, flies do not compensate for the lost sleep accrued during methamphetamine-induced waking (Fig. 2C) (14, 16, 21). As seen in Fig. 2D, Amylase expression was strongly activated by caffeine (filled bars) but not by methamphetamine (open bars). To determine whether Amylase levels are associated with naturally occurring conditions where sleep drive is high, we evaluated its progression during the first few days of the flies' adult life. Flies, like humans, exhibit ontogenetic variations in brain plasticity that are associated with increased sleep time (21, 22). As seen in Fig. 2E, daytime sleep was high immediately after eclosion and declined to adult levels by 3 days of age. Interestingly, the levels of Amylase followed these naturally occurring changes in sleep time (Fig. 2F). Together, these data indicate that Amylase is not simply a marker for waking but also for conditions where sleep drive is elevated.
Fig. 2.
Amylase is up-regulated during waking conditions associated with sleepiness. (A) tim01 flies show a minimal sleep rebound after 3 and 6 h of SD (open bars) but generate a sleep rebound typical of other clock mutants after 9 and 12 h of SD (filled bars). Percentage of sleep recovered is calculated as a ratio of the amount of sleep recovered divided by that lost. (B) Amylase mRNA levels remain low after deprivations that do not activate homeostatic mechanisms (3 and 6 h SD; open bars) but are elevated after deprivations that activate homeostatic mechanisms (9 and 12 h SD; filled bars). (C) Waking induced by methamphetamine (meth; 1 mg/ml; open bars) did not induce a homeostatic response, whereas waking induced by caffeine (2.5 mg/ml; filled bars) exhibited a rebound of similar magnitude as that seen after manual SD. (D) Amylase is elevated after caffeine administration but not after waking induced by methamphetamine. (E) Daytime sleep is highest in young flies and declines to stable adult values by 3 days of age. (F) Amylase levels decline with sleep time. Data are presented as percentage of 5-day-old flies.
To determine whether Amylase could provide an assessment of sleep drive in real time, we evaluated a Drosophila line in which the promoter for Amylase was linked to the firefly luciferase (Amy/Luc) (23). As shown in Fig. 3, bioluminescence was high after 16 h of SD and declined during 12 h of recovery (squares). In contrast, bioluminescence was low in siblings that had a full nights' sleep (circles) but increased as these flies spent time on caffeine consistent with the effects of caffeine on sleep homeostasis described above (14, 21). Thus, changes in bioluminescence are correlated with rising and falling levels of sleep drive in real time.
Fig. 3.
Amylase can provide an assessment of sleep drive in real-time. (A) Bioluminescence (counts/min ± SEM) in Amy/Luc flies is high immediately after 12 h of SD (n = 9) and declines during recovery (squares). In contrast, Amy/Luc flies that have slept all night and are placed onto caffeine (2.5 mg/ml) in the morning (n = 9) show a progressive increase in bioluminescence as sleep debt accrues (circles). Flies were maintained on 0.5% sucrose supplemented with 1 mM beetle luciferin. (B) Flies null for amylase display normal sleep time and sleep consolidation (average sleep bout duration (n = 32/group). (C) amy-null flies exhibit wild-type homeostatic response after SD; data indicate cumulative sleep lost then gained after 6 h of SD. A negative slope indicates sleep lost, and a positive slope indicates sleep gained; when the slope is zero recovery is complete.
Our data indicate that Amylase is highly correlated with sleep drive, but we wondered whether it plays a more direct role in sleep regulation. In Drosophila, α-Amylase enzymes are encoded by a pair of genes located on the right arm of the second chromosome, amy-p and amy-d (24). As shown in Fig. 3B, flies carrying a null mutation for both amylase genes (amy-pn amy-dn) display normal sleep time and architecture. Moreover, amy-pn amy-dn flies respond to SD with a homeostatic response similar to wild-type flies, indicating that Amylase is not mechanistically involved in sleep regulation (Fig. 3C).
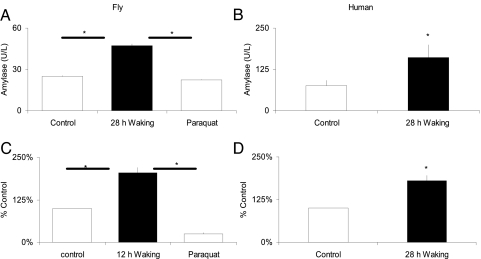
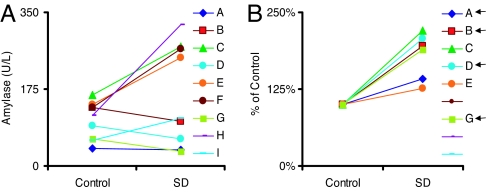
We wondered whether Amylase activity could be used as a biomarker for sleep drive in humans. Fig. 4A and B demonstrate that Amylase activity was increased in both humans and flies after 28 h of sustained waking compared with untreated circadian-matched controls; total salivary protein and volume were not significantly altered (P > 0.10; data not shown). In flies, paraquat did not alter Amylase activity, suggesting that these changes were not due to stress (Fig. 4A). Similarly, salivary cortisol was not altered by SD (0.254 ± 0.06 μg/dl) compared with untreated circadian-matched controls (0.251 ± 0.06 μg/dl), indicating that stress is also an unlikely explanation for the changes in Amylase in humans (n = 9). Recent reports indicate that mRNA from cell-free saliva extracts can be used to identify biomarkers of disease (25). Therefore, we evaluated Amylase mRNA levels in our sleep-deprived subjects. To ensure that the integrity of mRNA was consistent between subjects, only samples with intact β-actin were evaluated (25). As shown in Fig. 4 C and D, Amylase mRNA was increased ≈2-fold in both humans and flies after SD. Together, these data indicate that, as a group, Amylase activity and mRNA levels are elevated by sustained waking in humans. Because individuals vary greatly in their response to sleep loss (12), it is important to present data from individual subjects. As shown in Fig. 5A, Amylase activity was numerically elevated in five of nine subjects. In contrast, all subjects with intact salivary mRNA showed numerically increased Amylase mRNA levels, including four subjects whose Amylase activity was not altered (Fig. 5B). Interestingly, data from both the fly and human suggest that Amylase mRNA is a more sensitive measurement of sleepiness than Amylase activity.
Fig. 4.
Amylase can be used as a marker in flies and humans. (A and B) Amylase activity, but not protein levels, is increased in homogenates from Drosophila heads after 28 h of enforced waking (n = 20) (A) and in saliva samples taken from normal healthy humans (n = 9; P < 0.05) (B). In flies, 20 μM paraquat did not alter Amylase activity. Human saliva samples were collected at the same circadian time over consecutive weekends where each subject served as his or her own untreated control. (C and D) Amylase mRNA is increased in homogenates from Drosophila heads after 28 h of enforced waking (n = 20) (C) and in saliva samples taken from normal healthy humans (n = 6) after 28 h of waking (D) (P < 0.05).
Fig. 5.
Individual differences. (A) Amylase activity was increased in five of nine subjects after 28 h of waking. (B) Amylase mRNA levels were increased in all six saliva samples with intact β-actin. Arrows designate subjects with Amylase activity levels that were not elevated but that displayed increases in Amylase mRNA.
Discussion
We have outlined a simple and effective strategy for identifying biomarkers of sleepiness by using unique genetic and pharmacological tools in Drosophila. With this approach, it is possible to evaluate the behavior of a candidate molecule under a variety of experimental conditions and determine whether it is consistently associated with high sleep drive or whether it is nonspecifically activated by a particular experimental protocol. In addition, we have shown that it is possible to evaluate biomarkers of sleepiness in human subjects from samples derived from a readily accessible biological fluid by using noninvasive procedures. More importantly, we have identified a molecule, Amylase, that is highly correlated with sleep drive in the fly and is also responsive to waking in humans. It is important to note that recent studies have emphasized that the utility of saliva as a diagnostic tool will likely require a panel of biomarkers to be effective (26). As such, our data demonstrating that 28 h of waking increased salivary Amylase activity and mRNA represent the first step in developing an effective method for detecting sleepiness in vulnerable populations.
A noninvasive peripheral marker of sleepiness may be useful as an independent evaluation of sleep phenotypes both in the laboratory and in the field where electrophysiological measurements are not feasible. For example, genetic studies have begun to identify mutations in humans, mice, and flies that alter sleep time and sleep homeostasis (27–30). Similarly, ethological studies have reported instances where animals can sustain waking for extended periods of time without exhibiting a homeostatic response [e.g., cetacean (31) and white-crown sparrow (32)]. Frequently, it is not clear whether a mutation or an adaptation has altered sleep need or whether it has disrupted sleep-regulatory mechanisms and thus simply degraded the ability of the animal to respond appropriately to sleep loss. To distinguish between these possibilities, it is necessary to evaluate outcome measurements that should be adversely affected by inadequate sleep (e.g., lifespan, learning, and memory) but are not them-selves dependent on sleep regulatory networks. These experiments are not only time-consuming and costly, they are also confounded by the possibility that the mutation/adaptation could adversely alter sleep and the outcome measurement independently. Although not definitive, a biomarker that is independent of sleep regulatory mechanisms may provide additional mechanistic insight into the nature of the mutation and/or adaptation. Interestingly, flies mutant for Amylase respond to SD with a homeostatic response similar to wild-type flies, indicating that Amylase is not mechanistically involved in sleep regulation. Thus, Amylase is suitable to evaluate whether animals that experience sustained waking accrue sleep debt or whether they have a reduced need for sleep.
A limitation of our human study is the lack of subjective measures of sleepiness and objective measurements of performance decrements. However, it should be noted that a single night of sleep loss is known to reliably produce subjective feelings of sleepiness, negative mood, cognitive impairment, reduced attention, and driving impairments equivalent to blood alcohol level of 0.1 g % (33–35). Moreover, increased Amylase activity and mRNA levels were observed after 28 h of waking, 4 more waking hours than what would be considered criminal for drivers who cause accidents in New Jersey under “Maggie's Law” (11). Thus, the 28 h of waking in this experiment is more than sufficient to increase sleepiness and impair performance. An additional concern is whether the changes we see in Amylase are due to increased sleepiness or to stress. In flies, paraquat did not alter Amylase activity or mRNA levels, suggesting that these changes were not due to stress. A previous study in humans found that shorter amounts of SD (<8 h) do not activate salivary Amylase activity (36), and cortisol levels were unchanged after 28 h in our experiment. Thus, it is unlikely that stress plays a role in modifying Amylase during sustained periods of waking in either humans or flies.
Finally, one can ask whether Amylase will be effective in detecting sleepiness in individuals or whether it will only be effective using population measurements. This question is complicated by the growing awareness that individuals vary in their sensitivity to SD both in the extent to which they report being sleepy and in subsequent neurobehavioral deficits (12). These deficits are both task-specific and highly reproducible for a given subject, suggesting that they reflect trait characteristics. Although our sample size was not sufficient to evaluate individual differences directly, our data indicate that Amylase mRNA will be effective in measuring sleepiness in individuals. As seen in Fig. 5B, all subjects with intact salivary mRNA displayed numerical increases in Amylase after 28 h of waking. Whether the observed variability in Amylase mRNA levels will ultimately reflect individual trait characteristics in sensitivity to sleep loss awaits future investigations.
A major question about Drosophila sleep research is whether it has relevance for human sleep studies. We demonstrate here that 28 h of waking in human subjects significantly increased Amylase activity and mRNA levels compared with untreated, circadian-matched controls. Thus, a marker originally identified in flies is also modified by extended episodes of waking in humans. To our knowledge, results obtained in the fly and directly applied to human sleep research have not been reported previously. Thus, this work supports findings from other fields such as circadian rhythms, memory, and development that the fly can generate data that is immediately relevant for human studies.
Methods
Flies.
Flies were cultured at 25°C in 50–60% humidity for a 12 h:12 h light:dark cycle on yeast, dark corn syrup, and agar food as described (15). Lights came on at 8:00 a.m. cyc01;ry and yw;tim01flies were obtained from J. C. Hall (Brandeis University, Waltham, MA); Canton-S flies were obtained from the Bloomington Stock Center (Bloomington, IN), and Amy/Luc flies were obtained from Donnel Hickey (University of Ottawa, Ottawa, ON, Canada). Newly eclosed adult flies were collected from culture vials daily under CO2 anesthesia.
Procedure.
Three-day-old female flies were placed into 65-mm glass tubes, and sleep parameters were continuously evaluated throughout all experiments by using the Trikinetics activity monitoring system as described (refs. 15 and 21; see also www.Trikinetics.com). Flies were subjected to SD by using an automated SD apparatus that has been found to produce waking without nonspecifically activating stress responses (15, 21). Three independent replicates of 32 flies were conducted for each time point. After SD, two-thirds of the flies from each group were frozen, and RNA was extracted from whole heads. The remaining flies (one-third) were monitored for an additional 24 h to assay the size of the homeostatic response. By using this protocol, both gene expression and behavior can be evaluated in siblings that have been exposed to identical environments and experimental manipulations.
Pharmacological studies were conducted on female Canton-s flies. After 2 days of baseline recordings, flies were placed onto caffeine (2.5 mg/ml), methamphetamine (0.5 mg/ml), or vehicle 2 h before the beginning of their primary sleep period. The next morning, after 14 h of drug treatment, two-thirds of the flies from each group were frozen, and RNA was extracted from whole heads. The remaining flies were monitored for an additional 24 h to assay the size of the homeostatic response.
Amylase activity was evaluated after 28 h of SD in Cs flies to match the duration of waking used in the human experiments. Female Cs flies were sleep deprived for 28 h beginning at lights out (8:00 p.m.). Whole-body homogenates were extracted from four groups of five flies and compared with untreated circadian-matched controls; experiments were conducted in duplicate. Amylase activity was determined by using the Infinity Amylase reagent (TR25421; Thermo Electron Corp., Louisville, CO) according to the manufacturer's instructions. All samples were assayed in quadruplicate. To evaluate the effects of stress on Amylase activity, Cs females were placed onto 20 μM paraquat dissolved in 1% agar/5% sucrose for 16 h and killed at the same circadian time as the 28-h SD flies and their controls. We limited the duration on paraquat to 16 h because flies begin to die during longer exposure times (15).
Before luminescence recordings, sleep was evaluated for 2 days in female Amy/Luc flies as described above with the exception that they were maintained on 0.5% sucrose supplemented with 1 mM beetle luciferin (Promega, Madison Wisconsin). On day 3, flies were sleep deprived for 12 h (n = 9) or served as untreated controls (n = 9). Flies that were sleep deprived were removed from their 65-mm glass tubes and placed into Petri dishes with 200 μl of fresh 0.5% sucrose/1 mM beetle luciferin. Siblings that had not been sleep deprived were placed into Petri dishes with 2.5 mg/ml caffeine added to the 0.5% sucrose/1 mM beetle luciferin. Bioluminescence was subsequently recorded in 1-min bins for 12 h under photomultiplier tubes (HC135–11MOD; Hamamatsu, Shizouka, Japan) as described (37). Amy/Luc flies that had never been exposed to luciferin were recorded for 12 h and served as a blank. Luminescence for SD and caffeine-treated flies was subtracted from the blank and smoothed by using a 3-h running average. Two independent replicates were conducted.
Quantitative PCR.
Total RNA was isolated from fly heads by using TRIzol (Invitrogen, Carlsbad, CA) following the manufacturer's protocol. Reverse-transcription (RT) reactions were carried out in parallel on DNase I-digested total RNA as described (15). RT products were stored at −80°C until use. PCRs to measure levels of artificial transcript were performed to confirm uniformity of RT within sample groups and between samples. Comparable RTs within a sample group were pooled. All reverses were performed in quadruplicate. At least two quantitative PCR replications were performed for each condition. Values were expressed as a percentage of untreated animals and were evaluated by using one-way ANOVA.
Human Subjects.
Nine healthy human adult volunteers (seven men and two women) were enrolled in the study after providing their consent. The study was approved by the Institutional Review Board at Washington University School of Medicine.
Procedure.
The subjects were randomly separated in two groups, which where scheduled to alternate 2 weekends of either normal sleep or 28 h of continuous waking. The sleep protocol was carried out at the Sleep Medicine Center, Department of Neurology, Washington University School of Medicine. On the normal-sleep weekend, the volunteers were allowed to fall asleep at 10:00 p.m. Normal sleep architecture and absence of significant respiratory abnormalities during sleep, periodic limb movement disorder, parasomnias, and nocturnal seizures were confirmed by standard polysomnography. The polysomnograms were evaluated and scored following standard criteria (38). The SD group remained awake and was allowed free access to water during the night. However, meal times were restricted to 8:00 a.m., 12:00 noon, and 6:00 p.m. No efforts were taken to limit caffeine consumption during the day, and logs were kept for each participant. The participants were constantly monitored by two experienced, certified sleep technicians. Saliva was collected from plain (noncitric acid) cotton Salivettes (Sarstedt, Newton, NC) that had been chewed for ≈1 min. The samples were rapidly frozen over dry ice and kept at −80°C until assayed. Total protein was evaluated in saliva by using the Pyrogallol Red method (TP0400–1KT; Sigma, St. Louis, MO). Amylase activity was evaluated by Salimetrics Analytical Laboratory Services by using the Salivary α-Amylase Assay Kit (1-1902; Salimetrics, State College, PA). Saliva samples were assayed for cortisol by using a commercially available immunoassay protocol (Salimetrics). RNA was isolated from cell-free supernatant as described (19). RNA was treated with RNase-free DNase I (TURBO DNA-free; Ambion, Austin, TX) according to the manufacturer's instructions. Isolated RNA was reverse-transcribed by using SuperScript III (Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer's instructions. Quantitative PCR was performed by using the 7000 Real-Time PCR System (Applied Biosystems, Foster City, CA). Predesigned TaqMan Gene Expression Assays (Applied Biosystems) were used for analyzing the mRNA levels of β-actin and Amylase. A 9-μl aliquot of the cDNA was used in each reaction, and all reactions were performed in duplicate.
Acknowledgments
We thank Mathew Thimgan and David Van Essen for helpful comments and input; Michael Morrisey, Janine Kempleman, Min Quan, Lucy Vine, and Jordan Weitzner for assisting with the study; and David T. Wong for technical assistance. This work was supported by National Institutes of Health Grant R01-NS051305-01A1.
Abbreviations
- SD
sleep deprivation
- RT
reverse transcription.
Footnotes
The authors declare no conflict of interest.
References
- 1.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. J Appl Physiol. 2005;99:2008–2019. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 2.Quan SF. Am J Epidemiol. 2006;164:17–18. doi: 10.1093/aje/kwj200. and discussion 19–20. [DOI] [PubMed] [Google Scholar]
- 3.Akerstedt T, Peters B, Anund A, Kecklund G. J Sleep Res. 2005;14:17–20. doi: 10.1111/j.1365-2869.2004.00437.x. [DOI] [PubMed] [Google Scholar]
- 4.Petrie KJ, Powell D, Broadbent E. Ergonomics. 2004;47:461–468. doi: 10.1080/0014013031000085653. [DOI] [PubMed] [Google Scholar]
- 5.Taffinder NJ, McManus IC, Gul Y, Russell RC, Darzi A. Lancet. 1998;352:1191. doi: 10.1016/s0140-6736(98)00034-8. [DOI] [PubMed] [Google Scholar]
- 6.Rosekind MR. Sleep Med. 2005;6(Suppl 1):S21–S25. doi: 10.1016/s1389-9457(05)80005-x. [DOI] [PubMed] [Google Scholar]
- 7.Lowden A, Akerstedt T. Aviation Space Environ Med. 1998;69:596–602. [PubMed] [Google Scholar]
- 8.Lockley SW, Cronin JW, Evans EE, Cade BE, Lee CJ, Landrigan CP, Rothschild JM, Katz JT, Lilly CM, Stone PH, et al. N Engl J Med. 2004;351:1829–1837. doi: 10.1056/NEJMoa041404. [DOI] [PubMed] [Google Scholar]
- 9.Arnedt JT, Owens J, Crouch M, Stahl J, Carskadon MA. J Am Med Assoc. 2005;294:1025–1033. doi: 10.1001/jama.294.9.1025. [DOI] [PubMed] [Google Scholar]
- 10.Barger LK, Cade BE, Ayas NT, Cronin JW, Rosner B, Speizer FE, Czeisler CA. N Engl J Med. 2005;352:125–134. doi: 10.1056/NEJMoa041401. [DOI] [PubMed] [Google Scholar]
- 11.Jones CB, Dorrian J, Rajaratnam SM. Ind Health. 2005;43:63–70. doi: 10.2486/indhealth.43.63. [DOI] [PubMed] [Google Scholar]
- 12.Van Dongen HP, Vitellaro KM, Dinges DF. Sleep. 2005;28:479–496. doi: 10.1093/sleep/28.4.479. [DOI] [PubMed] [Google Scholar]
- 13.Dawson D, McCulloch K. Sleep Med Rev. 2005;9:365–380. doi: 10.1016/j.smrv.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, Sehgal A, Pack AI. Neuron. 2000;25:129–138. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 15.Shaw PJ, Tononi G, Greenspan RJ, Robinson DF. Nature. 2002;417:287–291. doi: 10.1038/417287a. [DOI] [PubMed] [Google Scholar]
- 16.Andretic R, van Swinderen B, Greenspan RJ. Curr Biol. 2005;15:1165–1175. doi: 10.1016/j.cub.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 17.Bosch JA, Ring C, de Geus EJ, Veerman EC, Amerongen AV. Int Rev Neurobiol. 2002;52:213–253. doi: 10.1016/s0074-7742(02)52011-0. [DOI] [PubMed] [Google Scholar]
- 18.Jansen AS, Ter Horst GJ, Mettenleiter TC, Loewy AD. Brain Res. 1992;572:253–260. doi: 10.1016/0006-8993(92)90479-s. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, St John MA, Zhou X, Kim Y, Sinha U, Jordan RC, Eisele D, Abemayor E, Elashoff D, Park NH, Wong DT. Clin Cancer Res. 2004;10:8442–8450. doi: 10.1158/1078-0432.CCR-04-1167. [DOI] [PubMed] [Google Scholar]
- 20.Hendricks JC, Lu S, Kume K, Yin JC, Yang Z, Sehgal A. J Biol Rhythms. 2003;18:12–25. doi: 10.1177/0748730402239673. [DOI] [PubMed] [Google Scholar]
- 21.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 22.Balling A, Technau GM, Heisenberg M. J Neurogenet. 1987;4:65–73. [PubMed] [Google Scholar]
- 23.Hickey DA, Benkel KI, Fong Y, Benkel BF. Proc Natl Acad Sci USA. 1994;91:11109–11112. doi: 10.1073/pnas.91.23.11109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hickey DA, Bally-Cuif L, Abukashawa S, Payant V, Benkel BF. Proc Natl Acad Sci USA. 1991;88:1611–1615. doi: 10.1073/pnas.88.5.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park NJ, Li Y, Yu T, Brinkman BM, Wong DT. Clin Chem. 2006;52:988–994. doi: 10.1373/clinchem.2005.063206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong DT. J Am Dent Assoc. 2006;137:313–321. doi: 10.14219/jada.archive.2006.0180. [DOI] [PubMed] [Google Scholar]
- 27.Hendricks JC, Williams JA, Panckeri K, Kirk D, Tello M, Yin JC, Sehgal A. Nat Neurosci. 2001;4:1108–1115. doi: 10.1038/nn743. [DOI] [PubMed] [Google Scholar]
- 28.Cirelli C, Bushey D, Hill S, Huber R, Kreber R, Ganetzky B, Tononi G. Nature. 2005;434:1087–1092. doi: 10.1038/nature03486. [DOI] [PubMed] [Google Scholar]
- 29.Franken P, Tafti M. Front Biosci. 2003;8:e381–e397. doi: 10.2741/1084. [DOI] [PubMed] [Google Scholar]
- 30.Mignot E. Sleep Med. 2004;5(Suppl 1):S2–S8. doi: 10.1016/s1389-9457(04)90001-9. [DOI] [PubMed] [Google Scholar]
- 31.Lyamin O, Pryaslova J, Lance V, Siegel J. Nature. 2005;435:1177. doi: 10.1038/4351177a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rattenborg NC, Mandt BH, Obermeyer WH, Winsauer PJ, Huber R, Wikelski M, Benca RM. PLoS Biol. 2004;2:E212. doi: 10.1371/journal.pbio.0020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banks S, Catcheside P, Lack LC, Grunstein RR, McEvoy RD. Sleep. 2005;28:1381–1385. doi: 10.1093/sleep/28.11.1381. [DOI] [PubMed] [Google Scholar]
- 34.Dawson D, Reid K. Nature. 1997;388:235. doi: 10.1038/40775. [DOI] [PubMed] [Google Scholar]
- 35.Rogers NL, Dorrian J, Dinges DF. Front Biosci. 2003;8:s1056–s1067. doi: 10.2741/1174. [DOI] [PubMed] [Google Scholar]
- 36.Parkkila S, Parkkila AK, Rajaniemi H. Acta Physiol Scand. 1995;154:205–211. doi: 10.1111/j.1748-1716.1995.tb09902.x. [DOI] [PubMed] [Google Scholar]
- 37.Abraham U, Prior JL, Granados-Fuentes D, Piwnica-Worms DR, Herzog ED. J Neurosci. 2005;25:8620–8626. doi: 10.1523/JNEUROSCI.2225-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rechtschaffen A, Kales A. A Manual of Standarized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Bethesda: US Dept of Health Education and Welfare, Public Health Service; 1968. [Google Scholar]