Abstract
Medical therapy is the preferred first-line approach in the management of lower urinary tract symptoms in men with benign prostatic hyperplasia. The magnitude of the improvement in lower urinary tract symptoms observed in response to combination therapy (α-blocker plus 5-α reductase inhibitors) does not approach that achieved with prostatectomy. Various drugs have been under consideration, including BXL628, lonidamine, and phosphodiesterase inhibitors, all of which have had unacceptable side effects. The gonadotropin-releasing hormone antagonist cetrorelix is associated with dose-dependent symptom improvement and reduction of prostate volume. Elucidating the mechanism for cetrorelix-mediated improvement in lower urinary tract symptoms will likely contribute to unraveling the pathophysiology of lower urinary tract symptoms in men.
Key words: Benign prostatic hyperplasia, Alpha-blockers, Lower urinary tract symptoms, 5-α reductase inhibitors, LH-RH antagonists, Cetrorelix
Over the past 20 years, medical therapy has gained an increasing role in the management of lower urinary tract symptoms (LUTS) arising from benign prostatic hyperplasia (BPH).1 Today, medical therapy is the preferred first-line approach to treating BPH. Alpha-blockers and 5-α reductase inhibitors (5-ARIs) are the 2 classes of drugs currently approved by the US Food and Drug Administration for the treatment of symptomatic BPH.
Alpha-blockers have been consistently shown to relieve LUTS in men with BPH, independent of prostate volume.2 Historically, nonselective α-blockers, such as phenoxybenzamine, were associated with adverse events.3 Over the past 20 years, the trend has been to develop α-blockers with improved tolerability. Tamsulosin and alfuzosin are currently the most widely prescribed α-blockers and are generally well tolerated. Side effects include asthenia, dizziness, headache, and ejaculatory dysfunction.
5-ARIs were initially shown to modestly improve LUTS in men with very large prostate glands.4 Side effects were limited to erectile and ejaculatory dysfunction. 5-ARIs fell into disfavor when Veterans Administration Cooperative Trial 359 demonstrated that finasteride and placebo were equally effective in relieving LUTS in men with symptomatic BPH.5 A meta-analysis subsequently demonstrated that the ability of 5-ARIs to relieve LUTS depended on prostate volume.6
The interest in 5-ARIs has been resurrected since the publication of results from the Medical Therapy of Prostate Symptoms (MTOPS) trial.7 Unlike all other multicenter, randomized, placebo-controlled trials assessing effectiveness, the primary endpoint of the MTOPS trials was BPH disease progression. In this study, BPH progression was defined as a 4-point increase in the American Urological Association (AUA) symptom score or the development of acute urinary retention (AUR), renal insufficiency, or incontinence. Both α-blockers and 5-ARIs significantly prevented disease progression through distinct mechanisms. Alpha-blockers primarily prevented symptom progression, whereas 5-ARIs prevented the development of AUR. 5-ARIs are now offered with the expectation that they will relieve LUTS and prevent AUR in men with enlarged prostate glands.
Are Additional Medical Therapies for BPH Needed?
There is agreement that currently available medical therapies significantly improve LUTS in men with BPH. Nevertheless, there is a substantial subset of men who do not tolerate or respond to medical therapy, and others experience disease progression while receiving medical therapy.7 The magnitude of the improvement in LUTS observed in response to combination therapy (α-blocker plus 5-ARI) does not approach the magnitude achieved with prostatectomy.5,8 Therefore, there is a definite need to develop novel medical therapies that target factors other than prostate smooth muscle relaxation or prostate volume reduction.
New Drugs in Development for BPH
Several new drugs are being developed for the treatment of BPH.
BXL628
The proliferation of prostate cells has been shown to be inhibited by the binding of agonists to vitamin D receptors.9 BXL628, an analogue of vitamin D3, has been shown in a rat model to inhibit proliferation of prostate cells by inducing apoptosis without impacting calcium hemostasis.10 In a pilot clinical study, BXL628 exhibited a significantly greater reduction of prostate volume compared with placebo after 12 weeks of active therapy.11 The effects of BXL628 on LUTS or bladder outlet obstruction were not reported. Longer and larger multicenter, randomized, placebo-controlled clinical trials are obviously required to support the utility of vitamin D receptor agonists for the treatment of BPH.
Lonidamine
Lonidamine is a novel agent that is a selective inhibitor of hexokinase, a pivotal enzyme for glycolysis.12 The prostate has been shown to be a relatively anaerobic organ.13 Therefore, its metabolism depends primarily on glycolysis. The high levels of citrate in the prostate serve as an inhibitor of the Krebs cycle, which makes the prostate even more dependent on glycolysis.14 Therefore, a selective inhibitor of glycolysis theoretically may exhibit a selective effect on prostate metabolism and function. Lonidamine has been shown to be effective when offered as combination therapy in some solid tumors, presumably because some tumors depend heavily on anaerobic metabolism.15 Ditonno and colleagues16 recently reported the safety and effectiveness of lonidamine in an open-label study of 45 men in Italy. After 12 weeks of treatment, statistically significant decreases were observed in mean prostate volume, mean serum levels of prostate-specific antigen, and mean AUA symptom score, along with a concomitant increase in the mean peak urinary flow rate (Qmax). A large phase III, randomized, double-blind, placebo-controlled study comparing lonidamine with placebo was recently terminated prematurely because of liver toxicity. An interim analysis failed to show treatment-dependent symptom improvement. Lonidamine is no longer being developed for BPH. The experience with lonidamine underscores the importance of randomized, placebo-controlled studies.
Phosphodiesterase-5 Inhibitors
Phosphodiesterase (PDE) inhibitors are widely used to treat erectile dysfunction.17 PDE type 5 (PDE-5) inhibitors prevent the breakdown of cyclic guanosine monophosphate, which enhances the effect of nitric oxide on penile smooth muscle. Nitric oxide synthase is present in the prostate.18 Nitric oxide has also been shown to mediate prostate smooth muscle tension.19 A recently reported study has demonstrated that PDE-5 inhibitors relieve LUTS in men with BPH.20 Interestingly, PDE-5 inhibitors improve LUTS without increasing Qmax. The mechanism of action of PDE-5 inhibitors on LUTS is poorly understood. PDE-5 inhibitors are associated with significant side effects, including flushing, gastroesophageal reflux, tachycardia, visual disturbances, and muscle cramps. The high cost of these drugs and their side effects, as well as the limited reduction of LUTS, renders this class of drugs a poor choice for treating BPH.
Gonadotropin-Releasing Hormone Antagonists for BPH
Gonadotropin-releasing hormone (GnRH) agonists are widely used for the treatment of prostate cancer.21 The primary limitation of GnRH agonists is the initial testosterone “flare,” which may be life threatening.22 The flare phenomenon may be prevented by pretreatment with antiandrogens.23 The delayed onset for achieving castrate levels in some cases and circumstances is problematic. The side effects of GnRH agonists include hot flashes, loss of libido, erectile dysfunction, osteoporosis, muscle wasting, and cognitive dysfunction.24 These side effects are acceptable when the goal is palliation of metastatic prostate cancer.
GnRH agonists were investigated for BPH in the 1990s. The side effects associated with castrate levels of testosterone were acceptable in relationship to the clinical benefit, which was only a modest improvement in LUTS.25
GnRH antagonists have the theoretical advantage over agonists for the treatment of both prostate cancer and BPH. An antagonist directly inhibits GnRH receptors and is not associated with the testosterone flare or delayed onset of testosterone suppression. A theoretical advantage of GnRH antagonists over agonists for the treatment of BPH is the ability to titrate the level of testosterone suppression. It is conceivable that an intermediate level of testosterone suppression may achieve therapeutic benefits at the level of the prostate while avoiding the typical adverse events associated with castrate levels of testosterone. Initial attempts to develop GnRH antagonists were limited by the occurrence of severe histamine-mediated anaphylactic reactions.
Abarelix was the first GnRH antagonist approved for the treatment of advanced prostate cancer.26 The abarelix package insert carries a black box warning for anaphylactic reactions. The drug cannot be administrated unless the patient signs a special consent form and resuscitative equipment is available in the office setting.27 It is no surprise that abarelix is rarely used in the treatment of prostate cancer. Abarelix was withdrawn from the US market because of limited sales.
Cetrorelix is the only GnRH antagonist that has been extensively studied in men with BPH. Cetrorelix is a decapeptide analogue of GnRH with substitutions of amino acids at positions 1, 2, 3, 6, and 10.28
The immediate-release formulation of cetrorelix acetate (Cetrotide®; Serono, Rockland, MA) has been approved for in vitro fertilization in more than 80 countries, including European nations, the United States, and Japan. Cetrotide has been marketed since 1999.
Cetrorelix acetate was the first formulation of the drug studied in men with BPH. The primary limitation of cetrorelix acetate was its short half-life, which required repeated subcutaneous (SC) injections and a large injection volume.
Gonzalez-Barcena and colleagues29 reported an open-label study of 11 men receiving 0.5 mg SC cetrorelix acetate twice daily for 28 days. A significant reduction in mean prostate volume, LUTS, and mean Qmax was observed. These clinical outcomes were maintained well beyond the 1-month active treatment phase. Similar results were obtained in a second open-label study.30
Lepor and colleagues31 reported the first multicenter, randomized, placebo-controlled clinical trial evaluating the safety and efficacy of cetrorelix acetate in men with symptomatic BPH. This proof-of-concept study was not adequately powered to demonstrate statistically significant outcomes. After a 7-day placebo lead-in, all subjects received daily SC treatment over a subsequent period of 28 days. The loading-dose group (CL) received 10 mg daily on days 1 through 5 and 1 mg daily on days 6 through 28. The other dose group (C) received 1 mg on days 1 through 28. The placebo group received daily injections of saline. On day 28, 32%, 52%, and 54% of men achieved at least a 30% decrease in symptom score in the placebo, C, and CL groups, respectively. The median increase in Qmax in the placebo, C, and CL groups was 1.0 mL/s, 3 mL/s, and 3 mL/s, respectively. The mean prostate volume decreased by 2.6 cm3, 6.2 cm3, and 7.1 cm3 in the placebo, C, and CL groups, respectively. Outcome measures were also evaluated 56 days after termination of active treatment. The effectiveness of cetrorelix acetate was durable. Subjects in the C group did not develop the typical side effects associated with castrate levels of testosterone.
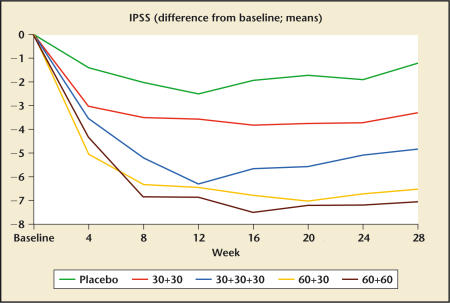
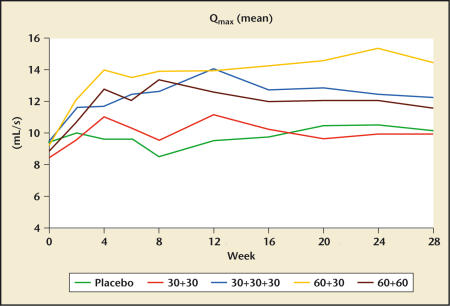
Debruyne and colleagues32 recently presented the results of a multicenter, randomized, double-blind, placebo-controlled study of cetrorelix pamoate in men with BPH. The longer half-life of the pamoate formulation allows for a more convenient dosing regimen. A total of 250 men were randomized into this study at several centers in Europe. After a 1-month single-blind placebo lead-in, subjects received placebo (P) or active drug (cet) on days 1, 14, and 28 according to the following dosing regimens: group I: P, P, P; group II: cet 30 mg, cet 30 mg, P; group III: cet 30 mg, cet 30 mg, cet 30 mg; group IV: cet 60 mg, cet 30 mg, P; and group V: cet 60 mg, cet 60 mg, P. All active-treatment groups experienced significant improvements in the mean AUA symptom score (Figure 1) and mean peak flow rate (Figure 2) relative to placebo at 12 weeks.
Figure 1.
Changes in International Prostate Symptom Score (IPSS) in response to placebo and 4 different dosing regimens of cetrorelix SR (pamoate) (Study Z003; dosing in week 0 and week 2; in group 30+30+30 also week 4). Data from Debruyne FMJ et al.32
Figure 2.
Changes in peak urinary flow rate (Qmax) in response to placebo and 4 different dosing regimens of cetrorelix SR (pamoate) (Study Z003; dosing in week 0 and week 2; in group 30+30+30 also week 4). Data from Debruyne FMJ et al.32
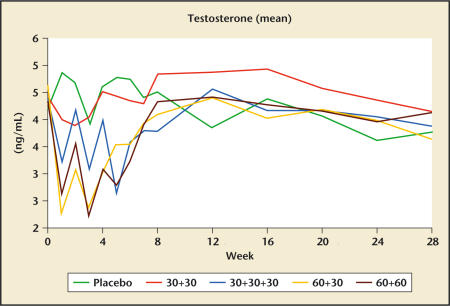
The time-dependent effect of cetrorelix on testosterone levels are shown in Figure 3. Even at the higher doses of cetrorelix, serum testosterone concentrations did not decline to a level approaching castrate levels. Testosterone levels returned to baseline values by 7 weeks. No significant differences were noted between active treatment and placebo for erectile function according to responses to the International Index of Erectile Function questionnaire, suggesting that the transient lowering of testosterone levels did not alter erectile function.
Figure 3.
Changes in serum testosterone levels in response to placebo and 4 different dosing regimens of cetrorelix SR (pamoate) (Study Z003; dosing in week 0 and week 2; in group 30+30+30 also week 4). Data from Debruyne FMJ et al.32
In the subgroup of men with enlarged prostate at baseline (prostate volume > 42 g) a significant decrease in prostate volume was noted between baseline and 4 weeks. Aeterna Zentaris (Québec, Canada) is planning to conduct 2 pivotal randomized placebo-controlled trials in Europe and the United States to support a new drug application for cetrorelix pamoate for the treatment of BPH. To date, the efficacy and tolerability data for cetrorelix in men with BPH is quite compelling.
The primary treatment goals for men with BPH are to alleviate LUTS and prevent progression to AUR. The pathophysiology of LUTS is poorly understood.33 There is increasing evidence that factors other than bladder outlet obstruction (BOO) and the extent of benign prostatic enlargement (BPE) are primarily responsible for the development of LUTS. There is also increasing evidence that α-blockers and 5-ARIs relieve LUTS by mechanisms other than prostate smooth muscle relaxation and prostate volume reduction, respectively.34 Therefore, it is unclear how to design a drug that is maximally effective at relieving LUTS. The risk of developing AUR is directly related to prostate volume.35 Therefore, drugs that reduce prostate volume have been shown to exhibit the greatest effect on preventing progression to AUR.7
The extensive clinical experience with cetrorelix in men with BPH has shown that this drug is associated with dose-dependent symptom improvement and reduction of prostate volume. On the basis of our current understanding of the pathophysiology of LUTS, it is unlikely that the reduction in prostate volume mediated by cetrorelix is directly responsible for the symptom improvement. The prostate volume reduction is comparable to that achieved with 5-ARIs, suggesting that cetrorelix may have a favorable long-term effect on preventing progression to AUR.
Cetrorelix elicits many effects on various hormones and growth factors that may mediate prostate volume reduction. Cetrorelix elicits an immediate and dose-dependent suppression of GnRH, which lowers luteinizing hormone and follicle-stimulating hormone, resulting in an immediate decrease of testosterone and its metabolized products dihydrotestosterone and estradiol.36 The fact that an intermediate level of testosterone suppression can achieve prostate volume reduction without causing hot flashes, erectile dysfunction, and other adverse events associated with castrate levels of testosterone suggests that there are different androgen thresholds mediating these events.
Another possible explanation may be that cetrorelix mediates prostate volume reduction via its effects on other hormones and growth factors. Cetrorelix reduces estrogen levels as a direct consequence of lowering testosterone. Estrogens have a direct effect on stromal proliferation.37 Lowering estrogens may not only prevent the further growth of the prostate, but may also promote regression of established BPH.
Growth factors may also play an important role in mediating benign and malignant prostate proliferation. Cetrorelix also has been shown to reduce the amount of epidermal growth factor receptors38 and insulin-like growth factor-II39 in prostate cancer. Cetrorelix may mediate the observed prostate volume reduction by altering levels of growth factors and their receptors. The prostate has been shown to have luteinizing hormone-releasing hormone receptors.40),41 The multifactorial effect of cetrorelix on various hormones and growth factors may explain its durable clinical outcomes despite the normalization of testosterone levels.
The mechanism for the cetrorelix-mediated improvement in LUTS is difficult to explain because our understanding of the pathophysiology of LUTS in the aging male is poorly understood.32 The fact that surgically resecting or enucleating the hyperplastic adenoma dramatically improves LUTS implies that the prostate is primarily responsible for the development and maintenance of LUTS in the aging male. The specific prostatic factors mediating LUTS have been elusive. The mechanism of action of cetrorelix in BPH may be mediated through unrecognized direct and indirect effects on the prostate. Elucidating the mechanism for cetrorelix-mediated improvement in LUTS will likely contribute to unraveling the pathophysiology of LUTS in men.
Main Points.
Alpha-blockers and 5-α reductase inhibitors are the 2 classes of drugs currently approved by the US Food and Drug Administration for the treatment of symptomatic benign prostatic hyperplasia.
The degree of improvement in lower urinary tract symptoms observed in response to combination therapy (α-blocker plus 5-α reductase inhibitors) does not approach that achieved with prostatectomy.
The gonadotropin-releasing hormone antagonist cetrorelix is associated with dose-dependent lower urinary tract symptom improvement and reduction of prostate volume, with minimal side effects.
References
- 1.Lepor H, Lowe FC, et al. Evaluation and nonsurgical management of benign prostatic hyperplasia. In: Walsh PC, Retik AB, Vaughan ED, et al., editors. Campbell’s Urology. 8th ed. Philadelphia: WB Saunders; 2002. pp. 1337–1378. [Google Scholar]
- 2.Lepor H, Williford WO, Barry MJ, et al. The impact of medical therapy on bother due to symptoms, quality of life and global outcome, and factors predicting response. Veterans Affairs Cooperative Studies Benign Prostatic Hyperplasia Study Group. J Urol. 1998;160:1358–1367. [PubMed] [Google Scholar]
- 3.Caine M, Perlberg S, Meretyk S. A placebo-controlled double-blind study of the effect of phenoxybenzamine in benign prostatic obstruction. Br J Urol. 1978;50:551–554. doi: 10.1111/j.1464-410x.1978.tb06210.x. [DOI] [PubMed] [Google Scholar]
- 4.Gormley GJ, Stoner E, Bruskewitz RC, et al. The effect of finasteride in men with benign prostatic hyperplasia. N Engl J Med. 1992;327:1185–1191. doi: 10.1056/NEJM199210223271701. [DOI] [PubMed] [Google Scholar]
- 5.Lepor H, Williford WO, Barry MJ, et al. The efficacy of terazosin, finasteride, or both in benign prostatic hyperplasia. Veterans Affairs Cooperative Studies Benign Prostatic Hyperplasia Study Group. N Engl J Med. 1996;335:533–539. doi: 10.1056/NEJM199608223350801. [DOI] [PubMed] [Google Scholar]
- 6.Boyle P, Gould AL, Roehrborn CG. Prostate volume predicts outcome of treatment of benign prostatic hyperplasia with finasteride: meta-analysis of randomized clinical trials. Urology. 1996;48:398–405. doi: 10.1016/s0090-4295(96)00353-6. [DOI] [PubMed] [Google Scholar]
- 7.McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387–2398. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 8.Lepor H, Rigaud G. The efficacy of transurethral resection of the prostate in men with moderate symptoms of prostatism. J Urol. 1990;143:533–537. doi: 10.1016/s0022-5347(17)40012-7. [DOI] [PubMed] [Google Scholar]
- 9.Peehl DM, Shinghal R, Nonn L, et al. Molecular activity of 1.25 dihydroxyvitamin D3 in primary cultures of human prostatic epithelial cells revealed by cDNA microarray analysis. J Ster Biochem Mol Biol. 2004;92:131–141. doi: 10.1016/j.jsbmb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Crescioli C, Ferruzzi P, Caporali A, et al. Inhibition of prostate cell growth by BXL-628, a calcitriol analogue selected for a phase II clinical trial in patients with benign prostate hyperplasia. Eur J Endocrinol. 2004;150:591–603. doi: 10.1530/eje.0.1500591. [DOI] [PubMed] [Google Scholar]
- 11.Colli E, Rigatti P, Montorsi F, et al. BXL628, a novel vitamin D3 analog, arrests prostate growth in patients with benign prostatic hyperplasia: a randomized clinical trial. Eur Urol. 2006;4949:82–86. doi: 10.1016/j.eururo.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 12.Horidi A, Lehninger AL. Action of the antitumor and anti-spermatogenic agent lonidamine on electron transport in Ehrlich ascites tumor mitochondria. Arch Biochem Biophys. 1983;226:73–83. doi: 10.1016/0003-9861(83)90272-2. [DOI] [PubMed] [Google Scholar]
- 13.Brawer MK. Lonidamine: basic science and rationale for treatment of prostatic proliferative disorders. Rev Urol. 2005;7(suppl 7):S21–S26. [PMC free article] [PubMed] [Google Scholar]
- 14.Costello LC, Franklin RB. The intermediary metabolism of the prostate: a key to understanding the pathogenesis and progression of prostate malignancy. Oncology. 2000;59:269–282. doi: 10.1159/000012183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robustelli Dela Cuna G, Pedrazzoli P. Toxicity and clinical tolerance of lonidamine. Semin Oncol. 1991;18:18–22. [PubMed] [Google Scholar]
- 16.Ditonno P, Battaglia M, Selvaggio O, et al. Clinical evidence supporting the role of lonidamine for the treatment of BPH. Rev Urol. 2005;7(suppl 7):S27–S33. [PMC free article] [PubMed] [Google Scholar]
- 17.Berner MM, Kriston L, Harms A. Efficacy of PDE-5 inhibitors for erectile dysfunction. A comparative meta-analysis of fixed-dose regimen randomized controlled trials administering the International Index of Erectile function in broad-spectrum populations. Int J Impot Res. 2006;18:229–235. doi: 10.1038/sj.ijir.3901395. [DOI] [PubMed] [Google Scholar]
- 18.Burnett AL, Maguire MP, Chamness SL, et al. Characterization and localization of nitric oxide synthase in the human prostate. Urology. 1995;45:435–439. doi: 10.1016/S0090-4295(99)80012-0. [DOI] [PubMed] [Google Scholar]
- 19.Takeda M, Tang R, Shapiro E, et al. Effects of nitric oxide on human and canine prostates. Urology. 1995;45:440–446. doi: 10.1016/S0090-4295(99)80013-2. [DOI] [PubMed] [Google Scholar]
- 20.McVary K, Roehrborn C, Kaminetsky J, et al. The efficacy and safety of tadalafil administered once a day for lower urinary tract symptoms (LUTS) in men with benign prostatic hyperplasia (BPH) [abstract 696]; the 21st Annual Congress of the European Association of Urology; April 15–18, 2006; Paris, France. [Google Scholar]
- 21.Lepor H. Comparison of single-agent androgen suppression for advanced prostate cancer. Rev Urol. 2005;7(suppl 5):S3–S12. [PMC free article] [PubMed] [Google Scholar]
- 22.Waxman J, Man A, Hendry WF, et al. Importance of early tumor exacerbation in patients treated with long-acting analogues of gonadotropin releasing hormone for advanced prostate cancer. BMJ. 1985;291:1387–1388. doi: 10.1136/bmj.291.6506.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulze H, Senge T. Influence of different types of antiandrogens on luteinizing hormone releasing hormone analogue-induced, testosterone surge in patients with metastatic carcinoma of the prostate. J Urol. 1990;144:934–941. doi: 10.1016/s0022-5347(17)39625-8. [DOI] [PubMed] [Google Scholar]
- 24.Kumar RJ, Barqawi AL, Crawford ED. Adverse events associated with hormonal therapy for prostate cancer. Rev Urol. 2005;7(suppl 5):S37–S43. [PMC free article] [PubMed] [Google Scholar]
- 25.Eri LM, Tveter KJ. A prospective placebo-controlled study of the luteinizing hormone-releasing hormone agonist leuprolide as treatment for patients with benign prostatic hyperplasia. J Urol. 1993;150:359–364. doi: 10.1016/s0022-5347(17)35483-6. [DOI] [PubMed] [Google Scholar]
- 26.Trachtenberg J, Gittleman M, Steidle C, et al. A phase 3, multicenter, open label, randomized study of abarelix versus leuprolide plus daily antiandrogen in men with prostate cancer. J Urol. 2002;167:1670–1674. doi: 10.1097/00005392-200204000-00021. [DOI] [PubMed] [Google Scholar]
- 27.Praecis Pharmaceuticals. Plenaxis package insert. Waltham, MA: Praecis Pharmaceuticals; 2005. [Google Scholar]
- 28.Bajusz S, Kovacs M, Gazdag M, et al. Highly potent antagonists of luteinizing hormone-releasing hormone free of edematogenic effects. Proc Natl Acad Sci U S A. 1988;85:1637–1641. doi: 10.1073/pnas.85.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez-Barcena D, Vadillo-Buenfil M, Gomez-Orta F, et al. Responses to the antagonistic analog of LH-RH SB-75, cetrorelix in patients with benign prostatic hyperplasia and prostatic cancer. Prostate. 1994;24:84–92. doi: 10.1002/pros.2990240206. [DOI] [PubMed] [Google Scholar]
- 30.Comaru-Schally AM, Brannan W, Schally AV, et al. Efficacy and safety of luteinizing hormone releasing hormone antagonist cetrorelix in the treatment of symptomatic benign prostatic hyperplasia. J Clin Endocrinol Metab. 1998;83:3826–3861. doi: 10.1210/jcem.83.11.5231. [DOI] [PubMed] [Google Scholar]
- 31.Lepor H, Dixon C, Crawford DE, et al. A randomized double blind placebo controlled phase II study of the safety and efficacy of cetrorelix in men with BPH; the Annual Meeting of the American Urological Association; April 12–17, 1997; New Orleans, LA. [Google Scholar]
- 32.Debruyne FMJ, Tzvetkov M, Medverec Z, et al. Cetrorelix pamoate, an LHRH antagonist, in the treatment of BPH: randomized, placebo-controlled multicenter study; the 28th Congress of the Société Internationale d’Urologie; November 12–16, 2006; Cape Town, South Africa. [Google Scholar]
- 33.Lepor H. Pathophysiology of lower urinary tract symptoms in the aging male population. Rev Urol. 2005;7(suppl 7):S3–S11. [PMC free article] [PubMed] [Google Scholar]
- 34.Lepor H. The pathophysiology of lower urinary tract symptom in the aging male population. In: Lepor H, editor. Prostatic Diseases. Philadelphia: WB Saunders; 2000. pp. 163–168. [Google Scholar]
- 35.Roehrborn CG. Acute urinary retention: risks and management. Rev Urol. 2005;7(suppl 4):S31–S41. [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez-Barcena D, Vadillo-Buenfil M, Guerra-Arguero L. [Google Scholar]
- 37.Henderson D, Habenicht UF, Nishino Y, et al. Aromatase inhibitors and benign prostatic hyperplasia. J Biochem. 1986;25:867–875. doi: 10.1016/0022-4731(86)90318-3. [DOI] [PubMed] [Google Scholar]
- 38.Lamharzi N, Halmos G, Jungwirth A, Schally AV. Decrease in the level and mRNA expression of LH-RH and EGF receptors after treatment with LH-RH antagonist cetrorelix in DU-145 prostate tumor xenografts in nude mice. Int J Oncol. 1998;13:429–435. doi: 10.3892/ijo.13.3.429. [DOI] [PubMed] [Google Scholar]
- 39.Lamharzi N, Schally AV, Koppan M. Luteinizing hormone-releasing hormone (LH-RH) antagonist cetrorelix inhibits growth of DU-145 human androgen-independent prostate carcinoma in nude mice and suppresses the levels and mRNA expression of IGF-II in tumors. Regul Pept. 1998;77:185–192. doi: 10.1016/s0167-0115(98)00119-0. [DOI] [PubMed] [Google Scholar]
- 40.Halmos G, Arencibia JM, Schally AV, et al. High incidence of receptors for luteinizing hormone-releasing hormone (LHRH) and LHRH receptor gene expression in human prostate cancers. J Urol. 2000;163:623–629. [PubMed] [Google Scholar]
- 41.Bono AV, Salvadore M, Celato N. Gonadotropin-releasing hormone receptors in prostate tissue. Anal Quant Cytol Histol. 2002;24:221–227. [PubMed] [Google Scholar]