Abstract
Bladder cancer is a common malignancy in the United States. Although urine cytology is a useful adjunct in both diagnosis and follow-up and is highly sensitive for detecting high-grade tumors, it is limited by decreased sensitivity in detecting low-grade tumors, which constitute the majority of new diagnoses. Additional screening tests with high sensitivity and specificity for urothelial tumors of all grades are indicated to help improve the diagnostic ability of urine cytology as well as to reduce the need for frequent cystoscopies, especially in those with low-risk disease. Several assays have been developed, with the ImmunoCyt/uCyt+ test (DiagnoCure, Inc., Québec, Canada) being especially promising. Recent studies on the applicability and efficacy of ImmunoCyt/uCyt+ testing are reviewed, as are its sensitivity, specificity, and predictive value in the follow-up and screening of urothelial malignancies.
Key words: Bladder cancer, Cystoscopy, ImmunoCyt/uCyt+ test, Urothelial malignancies
Bladder cancer is a common malignancy in American men and women, with 61,420 cases and 13,060 deaths predicted for 2006 in the United States.1 Patients treated for urothelial carcinoma require rigorous follow-up, with cystoscopy recommended every 3 months for the first 2 years, every 6 months for the next 2 years, and annually thereafter.2 This translates into high health care costs as well as frequent discomfort and inconvenience for patients.3 Although urine cytology is a useful adjunct in both diagnosis and follow-up and is highly sensitive for detecting high-grade tumors (79%), it is limited by decreased sensitivity (26%) in detecting low-grade tumors, which make up the majority of new diagnoses.4 A recent literature review found that the sensitivity of cytology is from 20% to 53%, with a mean of 34%; specificity is from 83% to 99.7%, with a mean of 99%.3 Additional screening tests with high sensitivity for tumors of all grades are indicated to help improve the diagnostic ability of urine cytology and to perhaps reduce the need for frequent cystoscopies, especially in those with low-risk disease. Several assays have been developed to address this need, with the ImmunoCyt/uCyt+ test (DiagnoCure, Inc., Québec, Canada) being especially promising. This article will review recent studies on the applicability and efficacy of ImmunoCyt/uCyt+ testing, as well as its sensitivity, specificity, and predictive value in the follow-up and screening of urothelial malignancies.
The ImmunoCyt/uCyt+ Test
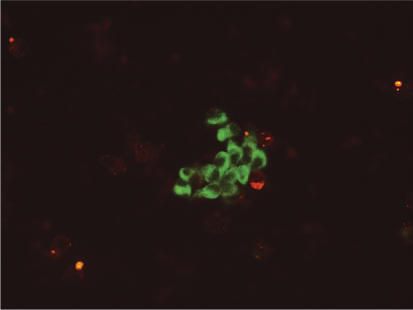
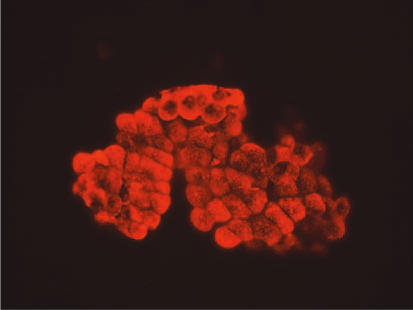
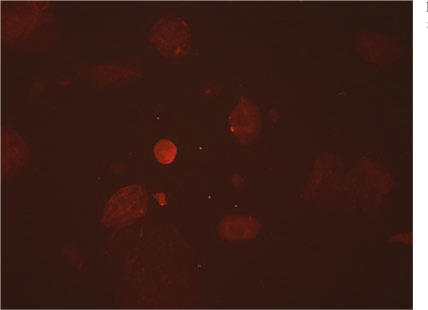
ImmunoCyt/uCyt+ is an immunocytochemical test developed by Fradet and Lockhard in 1997. It uses fluorescent-labeled antibodies to 3 markers that are commonly found on malignant exfoliated urothelial cells.5 One antibody is directed against a highmolecular-weight form of glycosylated carcinoembryonic antigen, 19A2115,6 and is labeled red. The other two antibodies, LDQ10 and M344,7 are directed against mucins, which are cytoplasmic antigens specific for bladder cancer and are labeled with fluorescein. Mucins are normally occurring, high-molecular-weight glycoproteins found on epithelial cell surfaces. In the case of urothelial malignancy, these glycoproteins are not as heavily glycosylated, thereby exposing a portion of the protein backbone. The antibodies LDQ10 and M344 are directed against new glycosylated epitopes.8,9 The tumor specificity of these antigens has been verified, with M344 expression being present in 71% of Ta-T1 tumors and 19A211 high-molecular-weight carcinoembryonic antigen expression found in 90% of Ta-T1 tumors.10 Red and green fluorescence is evaluated and quantified using a fluorescence microscope with a dual filter for fluorescein (the green marker) and Texas Red (the red marker). Examples are shown in Figures 1 and 2. A sample result is considered positive if at least 1 cell is seen to fluoresce green or red.8 A negative test result shows no fluorescence. An example of a negative test result is shown in Figure 3. The test is intended to be used on voided urine specimens in conjunction with cytologic analysis and increases overall sensitivity for all grades of tumor while maintaining the high specificity of conventional cytology.
Figure 1.
Positive Immunocyt/uCyt+ test result demonstrating green fluorescence.
Figure 2.
Positive Immunocyt/uCyt+ test result demonstrating red fluorescence.
Figure 3.
A negative Immunocyt/uCyt+ test result.
One constraint is that at least 500 cells without fluorescent signal must be observed on the slide before the sample can be called negative. Difficulty detecting low levels of green fluorescence and interference due to the red background have also been reported. These technical limitations suggest the need for proper training in performing the test and a learning curve with the assay. A study by Vriesema and colleagues on the reproducibility of the ImmunoCyt/uCyt+ test found high interobserver variability, with κ values between 0.05 and 0.45.8 A κ score of 1.0 indicates perfect agreement, whereas a κ less than 0.4 represents poor agreement.5,11 In a US multicenter study, Messing and colleagues12 found that 100% concordance could be achieved among pathologists through interobserver training and appropriate instruction in interpreting the assay. This study confirms the importance of adequate training and expertise among the cytotechnologists and cytopathologists who interpret the test. The use of proper equipment (eg, filters, mercury lamps), adequate slide preparation, and the use of positive and negative references with the assay are also important. The assay takes approximately 2 hours to complete from specimen filtration to slide preparation.13 Because this test must be interpreted by experienced cytopathologists in conjunction with urine cytology, the test cannot be used on site in the clinic, which is an option with some of the protein-based tests.
ImmunoCyt/uCyt+ in Follow-Up of Bladder Cancer
The use of the ImmunoCyt/uCyt+ test to detect recurrence of bladder cancer during surveillance has been well documented. The largest published study was by Mian and colleagues in 2006,14 in which 942 patients with a history of transitional cell carcinoma (TCC) of the bladder were enrolled. This study found that ImmunoCyt/uCyt+ had an increased sensitivity for low-grade tumors (G1), with the sensitivity being 8.3% for cytology alone compared with 79.3% for the combination of ImmunoCyt/uCyt+ and cytology. Sensitivity was improved for high-grade (G3) tumors as well, with a sensitivity of 75.3% for cytology alone and 98.9% for the combination of cytology and ImmunoCyt/uCyt+. Another multicenter study enrolled 694 patients: 458 were followed for TCC, and the remainder were new patients referred for suspicion of malignancy.15 Again, the addition of ImmunoCyt/uCyt+ to cytology improved sensitivity for low-grade (G1) tumors (from 17.9% to 66.7%) as well as for high-grade (G3) tumors (from 63.8% to 87%). Although the sensitivity of urinary cytology varied between the 10 study sites (27.3% to 68%), the combined sensitivity of ImmunoCyt/uCyt+ and cytology was higher, ranging from 57.1% to 90%. Messing and colleagues12 studied 341 patients with a history of TCC and confirmed these results, showing an increase in sensitivity for all grades and stages of tumor, including carcinoma in situ, when ImmunoCyt/uCyt+ was used with cytology for detection of recurrence. They concluded that the improved sensitivity of the Immuno-Cyt/uCyt+ test, especially in low-grade and low-stage tumors, may allow for a decrease in the frequency of follow-up cystoscopy for patients with negative cytology and Immuno-Cyt/uCyt+ examinations.
ImmunoCyt/uCyt+ in New Cases of Bladder Cancer
Several studies have examined the efficacy of ImmunoCyt/uCyt+ in patients who are newly referred to a urologist for evaluation of bladder cancer. Pfister and colleagues15 specifically examined the ability of ImmunoCyt/uCyt+ with and without cytology to screen for TCC in 236 new patients referred for suspicion of malignancy. Sensitivity for low-grade and low-stage tumors improved from 45.4% to 72.7% in these patients. This was comparable to the sensitivity of the test observed in patients with a history of TCC.15,16
Mian and colleagues17 reported a decreased positive predictive value with combined ImmunoCyt/uCyt+ and cytology in 107 new patients being evaluated for TCC, 93% to 55%; a negative predictive value of 90% in cytology; and 99% with the combined assay.17 The high negative predictive value suggests that the combination of tests is very reliable for ruling out the presence of bladder cancer, whereas the lower positive predictive value may indicate some false-positive test results. Lodde and colleagues11 confirmed these findings in 98 patients undergoing an initial evaluation for TCC. Again, sensitivity for all grades and stages of tumor was improved with the addition of the ImmunoCyt/uCyt+ assay to cytology alone. Sensitivity for G1 tumors increased from 5% to 85% and for pTa tumors from 13.8% to 86.2%. These values were similar to the results of the combination of tests observed in patients being followed for TCC.11 Furthermore, Lodde and colleagues13 studied 37 new patients being evaluated for upper tract TCC with ImmunoCyt/uCyt+ and cytology and found that the combination of tests improved sensitivity for all grades and stages of tumor, with 100% sensitivity compared with cytology alone. These data support the use of ImmunoCyt/uCyt+ and cytology in patients newly referred for suspicion of TCC of the upper or lower urinary tract.
Sensitivity, Specificity, and Predictive Value
Tables 1 through 4 detail the sensitivity, specificity, and positive and negative predictive values of ImmunoCyt/uCyt+ from 14 recent studies. In these studies, ImmunoCyt/uCyt+ was used in both follow-up for recurrent TCC and in new patients referred for evaluation of possible urothelial carcinoma. All cases were verified by cytology and cystoscopy. Patients were observed for lower and upper tract disease, and ImmunoCyt/uCyt+ performed very well regardless of location of urothelial tumor in 12 of 14 studies. In general, sensitivity ranged from 38.5% to 92.1% across all grades and risk categories of tumors. In almost all studies, ImmunoCyt/uCyt+ was more sensitive than standard voided cytology, with sensitivities from 23% to 84.6%. When the tests are used together, sensitivity improves a minimum of 15% over cytology alone, with a range in sensitivity between 53.8% and 94.1%. Specificity for ImmunoCyt/uCyt+ is inferior to cytology, with a range of 62% to 84.2% compared with 79.7% to 99.4% for cytology. When the tests are used in conjunction, overall specificity is slightly lower than that of cytology alone, with a range of 61% to 80.7%. ImmunoCyt/uCyt+ has a better negative predictive value than cytology (81% to 96.2% vs 86.4% to 89.7%) but a generally worse positive predictive value (26% to 63.2% vs 62.9% to 92.1%). This suggests that the ImmunoCyt/uCyt+ test has fewer false-negative results but more false-positive results than cytology alone. When used together, negative predictive value is superior to cytology alone, with a range of 90% to 97.3%. Positive predictive value is still inferior to cytology alone, however, but better than ImmunoCyt/uCyt+ alone, with a range of 29% to 82.7%.
Table 1.
Sensitivity of Urine Cytology, ImmunoCyt/uCyt+, and the Combination in Bladder Tumors of Various Grades
| Cyto | uCyt+ | Cyto | uCyt+ | Cyto | uCyt+ | ||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Cyto | uCyt+ | Combo | G1/Low | G1 | G2/Inter | G2 | G3/High | G3 |
| Lodde M et al 200113 | 50 | 75 | 87 | 0 | 33 | 17 | 100 | 100 | 71 |
| Piaton E et al 200316 | |||||||||
| New | 71.2 | 86.4 | 30 | 40 | 70.6 | 88.2 | 83.3 | 76.7 | |
| Follow-up | 55.2 | 79.3 | 38.1 | 61.9 | 58.3 | 66.7 | 64.1 | 76.9 | |
| Lodde M et al 200630 | 86.6 | 16.6 | 86.6 | 46.5 | 81.4 | 85.7 | 85.7 | ||
| Mian C et al 200331 | 45 | 86.2 | 90 | 6.4 | 80.6 | 45.8 | 87.5 | 92 | 92 |
| Lodde M et al 200311 | |||||||||
| New | 43.1 | 92.1 | 94.1 | 5 | 85 | 100 | 84.6 | 92.3 | |
| Follow-up | 39.2 | 82.3 | 86.2 | ||||||
| Feil G et al 200332 | 34.6 | 38.5 | 53.8 | 14.3 | 14.3 | 42.9 | 35.7 | 60 | 60 |
| Vriesema JL et al 20018 | 50 | ||||||||
| Mian C et al 199917 | 46.8 | 86.1 | 89.8 | 4 | 84 | 52 | 84 | 79.3 | 89.6 |
| Mian C et al 200614 | 38.9 | 84.9 | 89.3 | 8.3 | 79.3 | 43.3 | 84.1 | 75.3 | 92.1 |
| Pfister C et al 200315 | 48.9 | 66.7 | 75.9 | 17.9 | 60.7 | 46.3 | 75.6 | 63.8 | 76.8 |
| Toma MI et al 200433 | 84.6 | 78.3 | 89.1 | 85.7 | 85.7 | 87.0 | 73.9 | 75 | 83.3 |
| Messing EM et al 200512 | 23 | 81 | 81 | ||||||
| Tetu B et al 200518 | 29 | 74 | 84 | ||||||
| Hautmann S et al 200434 | 73 | 63.3 |
Values are percentages. Cyto, cytology; uCyt+, ImmunoCyt/uCyt+; Combo, cytology + ImmunoCyt/uCyt+; G1, grade 1; Low, low risk; G2, grade 2; Inter, intermediate risk; G3, grade 3; High, high risk.
Table 4.
Positive Predictive Value of Urine Cytology, ImmunoCyt/uCyt+, and the Combination in Bladder Tumors of Various Grades
| Cyto | uCyt+ | Combination | |
|---|---|---|---|
| Mian C et al 200614 | 92.1 | 36.7 | 37.9 |
| Toma MI et al 200433 | 75 | 63.2 | 65.1 |
| Tetu B et al 200518 | 70 | 26 | 29 |
| Hautmann S et al 200434 | 62.9 | 54.3 | |
| Mian C et al 200331 | 69.2 | ||
| Lodde M et al 200311 | |||
| New | 82.7 | ||
| Follow-up | 53.6 | ||
| Messing EM et al 200512 | 37 | ||
| Vriesema JL et al 20018 | 39 |
Values are percentages. Cyto, cytology; uCyt+, ImmunoCyt/uCyt+.
It is clear, based on these performance characteristics, that the ImmunoCyt/uCyt+ test can significantly improve the overall sensitivity of cytology alone for all grades of bladder tumor, and that it is best used to supplement cytology to exploit the enhanced specificity and positive predictive values yielded by the combination of tests. The hope is that the combination of high sensitivity with moderate specificity will allow some patients to prolong the interval between cystoscopies, especially those with low-grade and low-stage bladder tumors.15
The lower specificity seen with the ImmunoCyt/uCyt+ test approximates that of other urinary antigen-based diagnostic tests, such as the BTA TRAK and STAT tests (Polymedco, Inc., Cortlandt Manor, NY) and NMP22 (Matritech, Inc., Newton, MA). As in the case of the protein-based tests, this may be due to false-positive results generated in the setting of urinary tract infection, urinary lithiasis, and benign prostatic hyperplasia. Another explanation is that the false-positive results generated by the ImmunoCyt/uCyt+ test are actually an early detection of recurrence not yet clinically evident by cytology or cystoscopy, and that patients with a positive ImmunoCyt/uCyt+ test result in the absence of confirmatory cytology and cystoscopy are at higher risk for recurrence.14,16,18 These claims need further substantiation, however, before a seemingly false-positive ImmunoCyt/uCyt+ test result can be considered predictive of recurrence.
Comparison With Other Tests
Four additional urine-based diagnostic tests for recurrent bladder cancer are commercially available in the United States: the UroVysion fluorescence in situ hybridization assay (Vysis, Inc., Des Plaines, IL), BTA STAT, BTA TRAK, and NMP22. The UroVysion fluorescence in situ hybridization assay detects deletion of the 9p21 chromosomal region as well as amplification of chromosomes 3, 7, and 17. The sensitivity of the UroVysion assay has been reported at 36% to 95%; specificity, 89% to 96%.12,19 The BTA STAT and TRAK tests use monoclonal antibodies to detect complement factor H-related protein in voided urine. Sensitivity ranges between 58% and 72% and specificity between 48% and 75%.12–25 The NMP22 test uses a sandwich enzyme-linked immunosorbent assay with 2 monoclonal antibodies against the nuclear mitotic apparatus protein in urine and has reported sensitivity between 47% and 81% and specificity between 64.3% and 93.3%.26–29 A comparison between the sensitivity and specificity of these tests and ImmunoCyt/uCyt+ is provided in Table 5. In general, the combination ImmunoCyt/uCyt+ and cytology has similar specificity to the other commercially available tests but is more sensitive, especially for detection of low-grade/low-stage tumors. These performance characteristics make it appealing when combined with cytology to help prolong the interval between cystoscopies in patients followed for bladder cancer. It is also an attractive screening tool for patients referred with suspicion of bladder cancer, although no test to date can replace the gold standard of cystoscopy and cytology.
Table 5.
Comparison of Urinary Cancer Marker Assays
| Test | Sensitivity | Specificity |
|---|---|---|
| BTA STAT23–25 | 58–82.8 | 68–72 |
| BTA TRAK20–22 | 66–72 | 48–75 |
| NMP2226–29 | 47–81 | 67–93 |
| UroVysion FISH19,23 | 36–85 | 89–96 |
| ImmunocCyt/ | 53.8–94.1 | 62–84.2 |
| uCyt+ and | ||
| Cytology | ||
| Combination |
Values are percentages. FISH, fluorescence in situ hybridization.
In summary, ImmunoCyt/uCyt+ is an extensively tested assay with the ability to improve results obtained with cytology alone in the follow-up of patients with TCC of the bladder and upper urinary tract, especially in those patients with low-grade disease, which represents a large proportion of the patients being monitored for bladder cancer recurrence.
Main Points.
Although urine cytology is a useful adjunct in both diagnosis and follow-up and is highly sensitive for detecting high-grade urothelial tumors, it is limited by decreased sensitivity in detecting low-grade tumors, which make up the majority of new diagnoses.
Additional screening tests with high sensitivity and specificity for urothelial tumors of all grades are indicated to help improve the diagnostic ability of urine cytology and to reduce the need for frequent cystoscopies, especially in those with low-risk disease.
ImmunoCyt/uCyt+ is an extensively tested assay with the ability to improve results obtained with cytology alone in the follow-up of patients with transitional cell carcinoma of the bladder and upper urinary tract, especially in those patients with low-grade disease.
Table 2.
Specificity of Urine Cytology, ImmunoCyt/uCyt+, and the Combination in Bladder Tumors of Various Grades
| Study | Cyto | uCyt+ | Combo | CytoG1 | uCyt+ G1 | Cyto G2 | uCyt+ G2 | Cyto G3 | uCyt+ G3 |
|---|---|---|---|---|---|---|---|---|---|
| Piaton E et al 200316 | |||||||||
| New | 83.3 | 83.3 | |||||||
| Follow-up | 86.2 | 81.9 | |||||||
| Lodde M et al 200630 | 99 | 79.4 | 83.3 | 76.5 | 92 | 78 | |||
| Mian C et al 200331 | 94 | 71.3 | 65.6 | ||||||
| Lodde M et al 200311 | |||||||||
| New | 95 | 75 | 75 | ||||||
| Follow-up | 93.9 | 63.8 | 62.6 | ||||||
| Feil G et al 200332 | 91.9 | 83.9 | 81.6 | ||||||
| Vriesema JL et al 20018 | 73 | ||||||||
| Mian C et al 199917 | 98.2 | 79.4 | 79.4 | ||||||
| Mian C et al 200614 | 99.4 | 72.5 | 72.5 | ||||||
| Pfister C et al 200315 | 94.5 | 84.2 | 80.7 | ||||||
| Toma MI et al 200433 | 80 | 73.8 | 72.5 | ||||||
| Messing EM et al 200512 | 93 | 75 | 73 | ||||||
| Tetu B et al 200518 | 98 | 62 | 61 | ||||||
| Hautmann S et al 200434 | 79.7 | 75 |
Values are percentages. Cyto, cytology; uCyt+, ImmunoCyt/uCyt+; Combo, cytology + ImmunoCyt/uCyt+; G1, grade 1; G2, grade 2; G3, grade 3.
Table 3.
Negative Predictive Value of Urine Cytology, ImmunoCyt/uCyt+, and the Combination in Bladder Tumors of Various Grades
| Cyto | uCyt+ | Cyto | uCyt+ | Cyto | uCyt+ | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cyto | uCyt+ | Combo | Low/G1 | Low/G1 | Inter/G2 | Inter/G2 | High/G3 | High/G3 | |
| Mian C et al 200614 | 89.7 | 96.2 | 97.3 | ||||||
| Pfister C et al 200315 | 87.0 | 91.4 | 93.2 | ||||||
| Toma MI et al 200433 | 88 | 85.5 | 92.1 | ||||||
| Tetu B et al 200518 | 88 | 93 | 95 | ||||||
| Hautmann S et al 200434 | 86.4 | 81.3 | |||||||
| Vriesema JL et al 20018 | 81 | ||||||||
| Lodde M et al 200630 | 95.2 | 80.1 | 95.2 | 80.1 | 88.6 | 92 | 90.6 | ||
| Mian C et al 200331 | 90 | ||||||||
| Lodde M et al 200311 | |||||||||
| New | 90.1 | ||||||||
| Follow-up | 91.6 | ||||||||
| Messing EM et al 200512 | 95 |
Values are percentages. Cyto, cytology; uCyt+, ImmunoCyt/uCyt+; Combo, cytology + ImmunoCyt/uCyt+; Low, low risk; G1, grade 1; Inter, intermediate risk; G2, grade 2; High, high risk; G3, grade 3.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Smith JA , Jr, Labasky RF, Cockett AT, et al. Bladder cancer clinical guidelines panel summary report on the management of nonmuscle invasive bladder cancer (stages Ta, T1 and TIS). The American Urological Association. J Urol. 1999;162:1697–1701. [PubMed] [Google Scholar]
- 3.Lotan Y, Roehrborn CG. Cost-effectiveness of a modified care protocol substituting bladder tumor markers for cystoscopy for the followup of patients with transitional cell carcinoma of the bladder: a decision analytical approach. J Urol. 2002;167:75–79. [PubMed] [Google Scholar]
- 4.Bastacky S, Ibrahim S, Wilczynski SP, et al. The accuracy of urinary cytology in daily practice. Cancer. 1999;87:118–128. doi: 10.1002/(sici)1097-0142(19990625)87:3<118::aid-cncr4>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 5.Fradet Y, Lockhard C. Performance characteristics of a new monoclonal antibody test for bladder cancer: ImmunoCyt trade mark. Can J Urol. 1997;4:400–405. [PubMed] [Google Scholar]
- 6.Fradet Y, LaRue H, Parent-Vaugeois C, et al. Monoclonal antibody against a tumor-associated sialoglycoprotein of superficial papillary bladder tumors and cervical condylomas. Int J Cancer. 1990;46:990–997. doi: 10.1002/ijc.2910460607. [DOI] [PubMed] [Google Scholar]
- 7.Fradet Y, Islam N, Boucher L, et al. Polymorphic expression of a human superficial bladder tumor antigen defined by mouse monoclonal antibodies. Proc Natl Acad Sci U S A. 1987;84:7227–7231. doi: 10.1073/pnas.84.20.7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vriesema JL, Atsma F, Kiemeney LA, et al. Diagnostic efficacy of the ImmunoCyt test to detect superficial bladder cancer recurrence. Urology. 2001;58:367–371. doi: 10.1016/s0090-4295(01)01217-1. [DOI] [PubMed] [Google Scholar]
- 9.Bergeron A, Champetier S, LaRue H, et al. MAUB is a new mucin antigen associated with bladder cancer. J Biol Chem. 1996;271:6933–6940. doi: 10.1074/jbc.271.12.6933. [DOI] [PubMed] [Google Scholar]
- 10.Allard P, Fradet Y, Tetu B, et al. Tumor-associated antigens as prognostic factors for recurrence in 382 patients with primary transitional cell carcinoma of the bladder. Clin Cancer Res. 1995;1:1195–1202. [PubMed] [Google Scholar]
- 11.Lodde M, Mian C, Negri G, et al. Role of uCyt+ in the detection and surveillance of urothelial carcinoma. Urology. 2003;61:243–247. doi: 10.1016/s0090-4295(02)02073-3. [DOI] [PubMed] [Google Scholar]
- 12.Messing EM, Teot L, Korman H, et al. Performance of urine test in patients monitored for recurrence of bladder cancer: a multicenter study in the United States. J Urol. 2005;174:1238–1241. doi: 10.1097/01.ju.0000173918.84006.4d. [DOI] [PubMed] [Google Scholar]
- 13.Lodde M, Mian C, Wiener H, et al. Detection of upper urinary tract transitional cell carcinoma with ImmunoCyt: a preliminary report. Urology. 2001;58:362–366. doi: 10.1016/s0090-4295(01)01182-7. [DOI] [PubMed] [Google Scholar]
- 14.Mian C, Maier K, Comploj E, et al. uCyt+/ImmunoCyt in the detection of recurrent urothelial carcinoma: an update on 1991 analyses. Cancer. 2006;108:60–65. doi: 10.1002/cncr.21712. [DOI] [PubMed] [Google Scholar]
- 15.Pfister C, Chautard D, Devonec M, et al. Immunocyt test improves the diagnostic accuracy of urinary cytology: results of a French multicenter study. J Urol. 2003;169:921–924. doi: 10.1097/01.ju.0000048983.83079.4c. [DOI] [PubMed] [Google Scholar]
- 16.Piaton E, Daniel L, Verriele V, et al. Improved detection of urothelial carcinomas with fluorescence immunocytochemistry (uCyt+ assay) and urinary cytology: results of a French Prospective Multicenter Study. Lab Invest. 2003;83:845–852. doi: 10.1097/01.lab.0000074893.70675.2e. [DOI] [PubMed] [Google Scholar]
- 17.Mian C, Pycha A, Wiener H, et al. Immunocyt: a new tool for detecting transitional cell cancer of the urinary tract. J Urol. 1999;161:1486–1489. doi: 10.1016/s0022-5347(05)68934-3. [DOI] [PubMed] [Google Scholar]
- 18.Tetu B, Tiguert R, Harel F, et al. ImmunoCyt/uCyt+ improves the sensitivity of urine cytology in patients followed for urothelial carcinoma. Mod Pathol. 2005;18:83–89. doi: 10.1038/modpathol.3800262. [DOI] [PubMed] [Google Scholar]
- 19.Bubendorf L, Grilli B, Sauter G, et al. Multiprobe FISH for enhanced detection of bladder cancer in voided urine specimens and bladder washings. Am J Clin Pathol. 2001;116:79–86. doi: 10.1309/K5P2-4Y8B-7L5A-FAA9. [DOI] [PubMed] [Google Scholar]
- 20.Ellis WJ, Blumenstein BA, Ishak LM, et al. Clinical evaluation of the BTA TRAK assay and comparison to voided urine cytology and the Bard BTA test in patients with recurrent bladder tumors. The Multi Center Study Group. Urology. 1997;50:882–887. doi: 10.1016/s0090-4295(97)00508-6. [DOI] [PubMed] [Google Scholar]
- 21.Heicappell R, Wettig IC, Schostak M, et al. Quantitative detection of human complement factor H-related protein in transitional cell carcinoma of the urinary bladder. Eur Urol. 1999;35:81–87. doi: 10.1159/000019822. [DOI] [PubMed] [Google Scholar]
- 22.Thomas L, Leyh H, Marberger M, et al. Multicenter trial of the quantitative BTA TRAK assay in the detection of bladder cancer. Clin Chem. 1999;45:472–477. [PubMed] [Google Scholar]
- 23.Sarosdy MF, Hudson MA, Ellis WJ, et al. Improved detection of recurrent bladder cancer using the Bard BTA stat Test. Urology. 1997;50:349–353. doi: 10.1016/s0090-4295(97)00292-6. [DOI] [PubMed] [Google Scholar]
- 24.Wiener HG, Mian C, Haitel A, et al. Can urine bound diagnostic tests replace cystoscopy in the management of bladder cancer? J Urol. 1998;159:1876–1880. doi: 10.1016/S0022-5347(01)63184-7. [DOI] [PubMed] [Google Scholar]
- 25.Pode D, Shapiro A, Wald M, et al. Noninvasive detection of bladder cancer with the BTA stat test. J Urol. 1999;161:443–446. [PubMed] [Google Scholar]
- 26.Soloway MS, Briggman V, Carpinito GA, et al. Use of a new tumor marker, urinary NMP22, in the detection of occult or rapidly recurring transitional cell carcinoma of the urinary tract following surgical treatment. J Urol. 1996;156:363–367. doi: 10.1097/00005392-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Miyanaga N, Akaza H, Ishikawa S, et al. Clinical evaluation of nuclear matrix protein 22 (NMP22) in urine as a novel marker for urothelial cancer. Eur Urol. 1997;31:163–168. doi: 10.1159/000474443. [DOI] [PubMed] [Google Scholar]
- 28.Landman J, Chang Y, Kavaler E, et al. Sensitivity and specificity of NMP-22, telomerase, and BTA in the detection of human bladder cancer. Urology. 1998;52:398–402. doi: 10.1016/s0090-4295(98)00219-2. [DOI] [PubMed] [Google Scholar]
- 29.Hughes JH, Katz RL, Rodriguez-Villanueva J, et al. Urinary nuclear matrix protein 22 (NMP22): a diagnostic adjunct to urine cytologic examination for the detection of recurrent transitional-cell carcinoma of the bladder. Diagn Cytopathol. 1999;20:285–290. doi: 10.1002/(sici)1097-0339(199905)20:5<285::aid-dc7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 30.Lodde M, Mian C, Comploj E, et al. uCyt+ test: alternative to cystoscopy for less-invasive follow-up of patients with low risk of urothelial carcinoma. Urology. 2006;67:950–954. doi: 10.1016/j.urology.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 31.Mian C, Lodde M, Comploj E, et al. Liquid-based cytology as a tool for the performance of uCyt+ and Urovysion Multicolour-FISH in the detection of urothelial carcinoma. Cytopathology. 2003;14:338–342. doi: 10.1046/j.0956-5507.2003.00094.x. [DOI] [PubMed] [Google Scholar]
- 32.Feil G, Zumbragel A, Paulgen-Nelde HJ, et al. Accuracy of the ImmunoCyt assay in the diagnosis of transitional cell carcinoma of the urinary bladder. Anticancer Res. 2003;23:963–967. [PubMed] [Google Scholar]
- 33.Toma MI, Friedrich MG, Hautmann SH, et al. Comparison of the ImmunoCyt test and urinary cytology with other urine tests in the detection and surveillance of bladder cancer. World J Urol. 2004;22:145–149. doi: 10.1007/s00345-003-0390-8. [DOI] [PubMed] [Google Scholar]
- 34.Hautmann S, Toma M, Lorenzo Gomez MF, et al. Immunocyt and the HA-HAase urine tests for the detection of bladder cancer: a side-by-side comparison. Eur Urol. 2004;46:466–471. doi: 10.1016/j.eururo.2004.06.006. [DOI] [PubMed] [Google Scholar]