Abstract
Introduction
The aim of this study was to determine incidence, risk factors, and impact on outcome of intensive care unit (ICU)-acquired Stenotrophomonas maltophilia.
Methods
This prospective observational case-control study, which was a part of a cohort study, was conducted in a 30-bed ICU during a three year period. All immunocompetent patients hospitalised >48 hours were eligible. Patients with non-fermenting Gram-negative bacilli (NF-GNB) at ICU admission were excluded. Patients without ICU-acquired S. maltophilia who developed an ICU-acquired NF-GNB other than S. maltophilia were also excluded. Screening (tracheal aspirate and skin, anal, and nasal swabs) for NF-GNB was performed in all patients at ICU admission and weekly. Univariate and multivariate analyses were performed to determine risk factors for ICU-acquired S. maltophilia and for ICU mortality.
Results
Thirty-eight (2%) patients developed an S. maltophilia ICU-acquired colonisation and/or infection and were all successfully matched with 76 controls. Chronic obstructive pulmonary disease (COPD) and duration of antibiotic treatment (odds ratio [OR] [95% confidence interval (CI)] = 9.4 [3 to 29], p < 0.001, and 1.4 [1 to 2.3], p = 0.001, respectively) were independently associated with ICU-acquired S. maltophilia. Mortality rate (60% versus 40%, OR [95% CI] = 1.3 [1 to 1.7, p = 0.037]), duration of mechanical ventilation (23 ± 16 versus 7 ± 11 days, p < 0.001), and duration of ICU stay (29 ± 21 versus 15 ± 17 days, p < 0.001) were significantly higher in cases than in controls. In addition, ICU-acquired infection related to S. maltophilia was independently associated with ICU mortality (OR [95% CI] = 2.8 [1 to 7.7], p = 0.044).
Conclusion
COPD and duration of antibiotic treatment are independent risk factors for ICU-acquired S. maltophilia. ICU-acquired S. maltophilia is associated with increased morbidity and mortality rates. ICU-acquired infection related to S. maltophilia is an independent risk factor for ICU mortality.
Introduction
Non-fermenting Gram-negative bacilli (NF-GNB) colonisation and infection are frequent among critically ill patients [1-3]. Antibiotic resistance is common in NF-GNB, resulting in inappropriate initial antibiotic treatment and poor outcomes [4-6]. Several studies have investigated incidence, risk factors, and outcome of patients with Pseudomonas aeruginosa and Acinetobacter baumannii colonisation and infection. However, few studies have investigated patients with colonisation and/or infection related to Stenotrophomonas maltophilia.
Isolation rates of S. maltophilia have been increasing since the early 1970s, according to reports from several centres [7]. This pathogen primarily affects patients with co-morbid illness such as cystic fibrosis, immunosuppression, organ transplantation, and malignancies [8]. Infections related to S. maltophilia are associated with high morbidity and mortality rates. Therapy for these infections represents a significant challenge both for the clinician and the microbiologist because of this organism's high level of antibiotic resistance to most of the currently used agents and methodological difficulties in susceptibility testing with this organism [9,10].
In recent years, several trials have elucidated risk factors for S. maltophilia infection, which include neutropenia, presence of a central venous catheter, prolonged hospitalisation, and previous antibiotic treatment with broad-spectrum antibiotics [11-16]. However, only one study was performed in intensive care unit (ICU) patients with ventilator-associated pneumonia (VAP) related to S. maltophilia [16]. In addition, no study has evaluated risk factors for and impact on outcome of ICU-acquired S. maltophilia. Identifying risk factors for S. maltophilia colonisation and/or infection could be useful for future interventional studies aiming at preventing ICU-acquired S. maltophilia. Therefore, we conducted this study to determine incidence of, risk factors for, and outcome of ICU-acquired S. maltophilia.
Materials and methods
This prospective case-control study, which was a part of a cohort study, was conducted in a 30-bed medical and surgical ICU from January 1998 to January 2001. Because the study was observational, Institutional Review Board approval was not required; this was in accordance with Institutional Review Board regulation.
All patients who were without severe immunosuppression and who were hospitalised more than 48 hours in the ICU were eligible. Patients with colonisation and/or infection related to NF-GNB at ICU admission and patients without screening for NF-GNB colonisation at ICU admission and more than 48 hours after ICU admission were excluded. Patients without ICU-acquired S. maltophilia who developed an ICU-acquired colonisation and/or infection related to NF-GNB other than S. maltophilia were also excluded. However, patients with ICU-acquired colonisation and/or infection related to S. maltophilia and ICU-acquired colonisation and/or infection related to NF-GNB other than S. maltophilia were studied as cases.
Infection control policy included continuous surveillance of nosocomial infections, isolation techniques, and routine screening of multi-drug-resistant (MDR) bacteria. At ICU admission, isolation techniques were used in all patients until receipt of screening results. Thereafter, these techniques were performed in all patients with colonisation or infection related to MDR bacteria. Isolation techniques included use of protective gowns and gloves and adequate hand-washing with antiseptic soap between patient contacts. No selective digestive decontamination was performed. Antibiotic treatment was based on local antibiotic guidelines, including specific recommendations for each type of infection.
Routine screening of NF-GNB was performed in all patients at ICU admission and on a weekly basis (every Monday) thereafter. This screening included nasal, anal, and axilla swabs. In addition, tracheal aspirate was performed in intubated or tracheotomised patients. Hemocultures, quantitative tracheal aspirates, broncho-alveolar lavage, urine cultures, and other microbiologic cultures were performed according to clinical status.
Infection and colonisation were considered to be ICU-acquired if they were diagnosed more than 48 hours after ICU admission. VAP was defined by the presence of new or progressive radiographic infiltrate associated with two of the following criteria: temperature above 38.5°C or below 36.5°C, leucocyte count above 10,000/μl or below 1,500/μl, purulent tracheal aspirate, and positive (≥106 colony-forming units per ml) tracheal aspirate. Ventilator-associated tracheobronchitis was defined using all of the following criteria: fever (>38°C) with no other recognisable cause, new or increased sputum production, positive (≥106 colony-forming units per ml) endotracheal aspirate culture yielding a new bacteria, and no radiographic evidence of new pneumonia [17]. Other definitions of nosocomial infections were based on criteria of the CDC (Centers for Disease Control and Prevention) [18]. Colonisation was defined as a positive microbiologic culture without clinical signs of infection. Prior antibiotic treatment was defined as any antibiotic treatment during the two weeks preceding ICU admission. Severe immunosuppression was defined by the presence of neutropenia (leucocyte count <1,000/μl or neutrophils <500/μl), active solid or hematology malignancy, long-term corticosteroid therapy (≥1 mg/kg per day for >1 month), or HIV infection (CD4 <50/μl during the previous 6 months). Cases were patients with ICU-acquired S. maltophilia colonisation and/or infection. Controls were patients without NF-GNB colonisation and/or infection. Antibiotics used during the study period included glycopeptides (vancomycin and teicoplanin), extended-spectrum penicillins (amoxicillin/clavulanate, ticarcillin, ticarcillin/clavulanate, piperacillin, piperacillin/tazobactam, and imipenem), fluoroquinolones (ofloxacin and ciprofloxacin), extended-spectrum cephalosporins (cefotaxime, ceftriaxone, ceftazidime, and cefepime), aminoglycosides (gentamicin, tobramycin, and amikacin), and others (erythromycin, metronidazole, fusidic acid, rifampicin, and fosfomycin). Antimicrobial therapy was deemed inappropriate when none of the antibiotics used was active in vitro on S. maltophilia. Outcomes evaluated included ICU mortality, duration of mechanical ventilation, and duration of ICU stay.
Statistical methods
SPSS software (SPSS Inc., Chicago, IL, USA) was used for data analysis. Proportions were compared using the χ2 test or the Fisher exact test whereas appropriate, continuous variables were compared using the Student t test or Mann-Whitney U test. Results are presented as number (percentage) for frequency and as mean ± standard deviation for quantitative variables. Odds ratio (OR) [95% confidence interval (CI)] was calculated for all significant (p < 0.05) qualitative variables in univariate analysis and for all significant variables in multivariate analysis.
Every case patient was matched to two control patients according to all the following criteria: (a) duration of ICU stay before ICU-acquired S. maltophilia occurrence (controls ≥ cases), (b) Simplified Acute Physiology Score (SAPS) II at ICU admission ± 5 points, (c) age ± 5 years, (d) admission category (medical/surgical), and (e) date of ICU admission when more than two control patients were well matched to a case. The controls chosen were the ones with the closest date of admission to that of case patients.
Univariate and multivariate analyses were used to determine variables associated with ICU-acquired S. maltophilia. The following variables were included in univariate analysis: age, gender, SAPS II and organ failures on ICU admission, transfer from other wards, admission category (medical/surgical), diabetes mellitus, chronic obstructive pulmonary disease (COPD) [19], prior antibiotic treatment, central venous catheter, urinary catheter, tracheostomy, mechanical ventilation, duration of mechanical ventilation, duration of ICU stay, antibiotic treatment, and duration of antibiotic treatment. Treatment with the following antibiotics and its duration were also included in univariate analysis: glycopeptide, extended-spectrum penicillin, fluoroquinolones, extended-spectrum cephalosporin, carbapenem, aminoglycoside, and metronidazole. In patients with ICU-acquired S. maltophilia, only exposure to potential risk factors before S. maltophilia acquisition was taken into account.
To determine risk factors for ICU-acquired infection related to S. maltophilia, cases with only colonisation and their controls were excluded from risk factor analysis. Cases with an ICU-acquired infection and their controls were compared using univariate and multivariate analyses. All the above-cited variables were included in these analyses.
Univariate and multivariate analyses were performed to determine risk factors for ICU mortality among cases and controls. All the above-cited variables were included in univariate analysis. In addition, ICU-acquired S. maltophilia and ICU-acquired infection related to S. maltophilia were included in risk factor analyses of ICU mortality. Variables with p < 0.2 by univariate analysis were included in stepwise logistic regression models. Prior antibiotic use as well as antibiotic treatment in the ICU and its duration were compared between patients with COPD and patients without COPD.
Results
Patient characteristics
One thousand eight hundred and eighty-five patients were eligible, 25 (1%) patients were excluded for NF-GNB colonisation and/or infection at ICU admission, and 20 (1%) patients for absence of NF-GNB screening at ICU admission and more than 48 hours after ICU admission. Among the 1,840 remaining patients, 324 (17%) patients developed an ICU-acquired NF-GNB. Two hundred and eighty-six (15%) patients without ICU-acquired S. maltophilia were excluded for ICU-acquired colonisation and/or infection related to NF-GNB other than S. maltophilia. Thirty-eight (2%) patients developed an ICU-acquired colonisation and/or infection related to S. maltophilia, representing two patients with ICU-acquired S. maltophilia per 1,000 ICU days. These patients were all successfully matched with 76 control patients (Figure 1). Among cases, three patients had an ICU-acquired colonisation and/or infection related to NF-GNB other than S. maltophilia. Among cases with ICU-acquired infection related to S. maltophilia, 24 (80%) patients had prior colonisation related to S. maltophilia.
Figure 1.
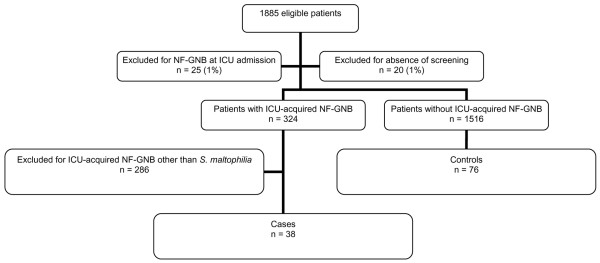
Profile of the study in this report. ICU, intensive care unit; NF-GNB, non-fermenting Gram-negative bacilli
The mean time between ICU admission and first ICU-acquired S. maltophilia was 14 ± 11 days. Thirty of 38 (78%) patients developed 36 ICU-acquired infections related to S. maltophilia, including 22 VAP, eight ventilator-associated tracheobronchitis, four urinary infections, and two bacteremias. Patient characteristics are presented in Table 1.
Table 1.
Risk factors for ICU-acquired Stenotrophomonas maltophilia and ICU-acquired infection related to S. maltophilia by univariate analysis
| Cases (n = 38) | Controls (n = 76) | p value | Cases with infection (n = 30) | Controls (n = 60) | p value | |
| At ICU admission | ||||||
| Age, years | 63 ± 12 | 65 ± 12 | 0.573 | 63 ± 13 | 65 ± 13 | 0.523 |
| SAPS II | 32 ± 15 | 30 ± 15 | 0.582 | 32 ± 15 | 31 ± 15 | 0.784 |
| Male gender | 24 (63) | 50 (65) | 0.470 | 10 (33) | 20 (33) | NA |
| Transfer to the ICU from a ward | 31 (81) | 65 (85) | 0.358 | 25 (83) | 50 (83) | NA |
| Surgery | 9 (23) | 18 (23) | NA | 7 (23) | 14 (23) | NA |
| Diabetes mellitus | 4 (10) | 7 (9) | 0.532 | 4 (13) | 6 (10) | 0.441 |
| COPD | 24 (63) | 17 (22) | <0.001a | 20 (66) | 12 (20) | <0.001b |
| Cystic fibrosis | 3 (7) | 2 (2) | 0.205 | 3 (10) | 1 (1) | 0.106 |
| Prior antibiotic treatment | 19 (50) | 29 (38) | 0.157 | 15 (50) | 23 (38) | 0.203 |
| Organ failure | ||||||
| Respiratory | 38 (100) | 53 (69) | <0.001c | 30 (100) | 39 (65) | <0.001d |
| Cardiac | 8 (18) | 13 (17) | 0.431 | 7 (23) | 9 (15) | 0.267 |
| Renal | 9 (23) | 16 (21) | 0.506 | 7 (23) | 13 (21) | 0.561 |
| Neurologic | 6 (25) | 24 (31) | 0.042e | 4 (13) | 20 (33) | 0.028f |
| Digestive | 1 (2) | 8 (10) | 0.120 | 1 (3) | 8 (13) | 0.119 |
| During ICU hospitalization | ||||||
| Central venous catheter | 35 (92) | 69 (90) | 0.559 | 27 (90) | 55 (91) | 0.537 |
| Urinary catheter | 35 (92) | 63 (82) | 0.147 | 28 (93) | 50 (83) | 0.162 |
| Tracheostomy | 6 (15) | 10 (13) | 0.453 | 4 (13) | 6 (10) | 0.441 |
| Mechanical ventilation | 32 (84) | 57 (75) | 0.191 | 30 (100) | 57 (95) | 0.442 |
| Duration of mechanical ventilation, days | 12 ± 10 | 7 ± 11 | 0.026 | 12 ± 10 | 8 ± 12 | 0.016 |
| Duration of ICU stay, days | 14 ± 11 | 15 ± 17 | 0.537 | 14 ± 12 | 14 ± 20 | 0.523 |
Data are presented as mean ± standard deviation or number (percentage). Odds ratio (95% confidence interval) = a1.9 (1.3 to 2.8), b2.2 (1.3 to 3.5), c1.7 (1.4 to 2), d1.7 (1.4 to 2.1), e2.6 (1 to 7), and f2.4 (1 to 6.2). COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; NA, not applicable; SAPS, Simplified Acute Physiology Score.
Risk factors for ICU-acquired S. maltophilia
Risk factors for ICU-acquired S. maltophilia and for ICU-acquired infection related to S. maltophilia which were determined by univariate analysis are presented in Tables 1 and 2. COPD (OR [95% CI] = 9.4 [3 to 29], p < 0.001) and duration of antibiotic treatment (OR [95% CI] = 1.4 per day [1 to 2.3], p = 0.001) were independently associated with ICU-acquired S. maltophilia. Antecedent COPD and duration of antibiotic treatment were also independently associated with ICU-acquired infection related to S. maltophilia (OR [95% CI] = 9.1 [2.5 to 32], p < 0.001, and 1.16 [1 to 1.2], p = 0.001, respectively).
Table 2.
Antibiotic use in study patients during intensive care unit stay
| Cases (n = 38) | Controls (n = 76) | p value | Cases with infection (n = 30) | Controls (n = 60) | p value | |
| Antibiotic treatment | 38 (100) | 47 (61) | <0.001a | 30 (100) | 36 (60) | <0.001b |
| Duration of antibiotic treatment, days | 13 ± 10 | 5 ± 6 | <0.001 | 16 ± 11 | 5 ± 6 | <0.001 |
| Glycopeptide use | 7 (18) | 12 (15) | 0.457 | 6 (20) | 10 (16) | 0.453 |
| Duration, days | 1 ± 4 | 1 ± 2 | 0.582 | 2 ± 4 | 1 ± 2 | 0.581 |
| Extended-spectrum penicillin use | 32 (84) | 38 (50) | <0.001c | 25 (83) | 28 (46) | 0.001d |
| Duration, days | 10 ± 9 | 4 ± 6 | <0.001 | 11 ± 10 | 4 ± 6 | 0.001 |
| Fluoroquinolone use | 17 (44) | 24 (31) | 0.121 | 12 (40) | 17 (28) | 0.190 |
| Duration, days | 4 ± 6 | 2 ± 3 | 0.050 | 4 ± 6 | 2 ± 4 | 0.124 |
| Extended-spectrum cephalosporin use | 12 (31) | 6 (7) | 0.002e | 9 (30) | 5 (83) | 0.010f |
| Duration, days | 3 ± 6 | 0.3 ± 1 | 0.001 | 4 ± 7 | 0.3 ± 1 | 0.004 |
| Carbapenem use | 6 (15) | 4 (5) | 0.067 | 4 (13) | 4 (6) | 0.251 |
| Duration, days | 4 ± 3 | 2 ± 1 | 0.058 | 1 ± 2 | 0.4 ± 2 | 0.294 |
| Aminoglycoside use | 11 (28) | 14 (18) | 0.149 | 10 (33) | 11 (18) | 0.095 |
| Duration, days | 2 ± 5 | 1 ± 3 | 0.154 | 3 ± 6 | 1 ± 3 | 0.080 |
| Metronidazole use | 3 (7) | 4 (5) | 0.429 | 1 (3) | 4 (6) | 0.457 |
| Duration, days | 0.5 ± 2 | 0.3 ± 2 | 0.573 | 0.3 ± 2 | 0.4 ± 2 | 0.532 |
Results by univariate analysis. Data are presented as mean ± standard deviation or number (percentage). Odds ratio (95% confidence interval) = a1.8 (1.4 to 2.1), b1.8 (1.4 to 2.2), c1.5 (1.2 to 2), d5.7 (1.9 to 16.9), e2.1 (1.1 to 4.2), and f4.7 (1.4 to 15.7).
Rates of prior antibiotic use (21 of 41 [51%] versus 27 of 73 [36%], p = 0.101) and antibiotic use during ICU stay (32 of 41 [78%] versus 53 of 73 [72%], p = 0.342) were similar in patients with COPD as compared with patients without COPD, respectively. Duration of antibiotic treatment during ICU stay was similar in patients with COPD and patients without COPD (11 ± 8 days versus 8 ± 6 days, p = 0.224).
Outcomes of study patients
ICU mortality rate, duration of mechanical ventilation, and duration of ICU stay were significantly higher in cases than in controls and were significantly higher in cases with S. maltophilia ICU-acquired infection than in their controls (Table 3).
Table 3.
Outcomes of study patients
| Cases (n = 38) | Controls (n = 76) | p value | Cases with infection (n = 30) | Controls (n = 60) | p value | |
| Duration of mechanical ventilation, days | 23 ± 16 | 7 ± 11 | <0.001 | 26 ± 17 | 8 ± 12 | <0.001 |
| Duration of ICU stay, days | 29 ± 21 | 15 ± 17 | <0.001 | 29 ± 19 | 14 ± 20 | <0.001 |
| ICU mortality | 23 (60) | 31 (40) | 0.037a | 21 (70) | 23 (38) | 0.004b |
Results by univariate analysis. Data are presented as mean ± standard deviation or number (percentage). Odds ratio (95% confidence interval) = a1.3 (1 to 1.7) and b3.7 (1.4 to 9.5). ICU, intensive care unit.
Although mortality rate was significantly higher in cases with S. maltophilia ICU-acquired infection than in cases with S. maltophilia ICU-acquired colonisation (21 of 30 [70%] versus 2 of 8 [25%], OR [95% CI] = 2.5 [1.2 to 4.9], p = 0.029), duration of mechanical ventilation (22 ± 18 days versus 10 ± 11 days, p = 0.104) and duration of ICU stay (29 ± 19 days versus 31 ± 30 days, p = 0.847) were similar in the two groups.
In cases with S. maltophilia ICU-acquired infection, mortality rate was significantly higher in patients who received inappropriate initial antibiotic treatment than in patients who received appropriate initial antibiotic treatment (8 of 8 [100%] versus 13 of 22 patients [59%], OR [95% CI] = 1.6 [1.1 to 2.3], p = 0.035).
Risk factors for ICU mortality
Risk factors for ICU mortality which wer determined by univariate analysis are presented in Tables 4 and 5. Multivariate analysis identified cardiac failure (OR [95% CI] = 52 [5 to 496], p = 0.001), S. maltophilia ICU-acquired infection (OR [95% CI] = 2.8 [1 to 7.7], p = 0.044), and respiratory failure (OR [95% CI] = 5 [1 to 25], p = 0.047) as independent risk factors for ICU mortality.
Table 4.
Risk factors for ICU mortality by univariate analysis
| Survivors (n = 60) | Non-survivors (n = 54) | p value | |
| At ICU admission | |||
| Age, years | 58 ± 19 | 66 ± 13 | 0.025 |
| SAPS II | 31 ± 16 | 32 ± 14 | 0.613 |
| Male gender | 36 (60) | 38 (70) | 0.168 |
| Transfer to the ICU from a ward | 47 (78) | 49 (91) | 0.058 |
| Surgery | 10 (17) | 17 (31) | 0.051 |
| Diabetes mellitus | 5 (8) | 6 (11) | 0.426 |
| COPD | 19 (32) | 22 (41) | 0.208 |
| Cystic fibrosis | 3 (5) | 2 (4) | 0.550 |
| Prior antibiotic treatment | 19 (32) | 29 (54) | 0.014a |
| Organ failure | |||
| Respiratory | 42 (70) | 49 (91) | 0.017b |
| Cardiac | 1 (2) | 20 (37) | <0.001c |
| Renal | 8 (13) | 17 (31) | 0.024d |
| Neurologic | 17 (28) | 13 (24) | 0.320 |
| Digestive | 5 (8) | 4 (7) | 0.534 |
| During ICU hospitalization | |||
| Central venous catheter | 55 (92) | 49 (91) | 0.560 |
| Urinary catheter | 51 (85) | 47 (87) | 0.484 |
| Tracheostomy | 9 (15) | 7 (13) | 0.484 |
| Mechanical ventilation | 43 (72) | 46 (85) | 0.064 |
| Duration of mechanical ventilation, days | 10 ± 14 | 15 ± 17 | 0.058 |
| Duration of ICU stay, days | 17 ± 19 | 21 ± 23 | 0.571 |
| ICU-acquired Stenotrophomonas maltophilia | 15 (25) | 23 (43) | 0.037e |
| ICU-acquired infection related to S. maltophilia | 9 (15) | 21 (39) | 0.004f |
Data are presented as mean ± standard deviation or number (percentage). Odds ratio (95% confidence interval) = a2.5 (1.1 to 5.3), b1.48 (1 to 2.1), c3.5 (1.1 to 10.4), d32.9 (4.2 to 256), e2.8 (1 to 7.2), and f3.6 (1.4 to 8.8). COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; SAPS, Simplified Acute Physiology Score.
Table 5.
Relationship between antibiotic use and intensive care unit mortality in univariate analysis
| Survivors (n = 60) | Non-survivors (n = 54) | p value | |
| Antibiotic treatment | 38 (63) | 47 (87) | <0.003a |
| Duration of antibiotic treatment, days | 7 ± 8 | 10 ± 10 | 0.197 |
| Glycopeptide use | 9 (15) | 11 (20) | 0.225 |
| Duration, days | 2 ± 3 | 1 ± 3 | 0.399 |
| Extended-spectrum penicillin use | 34 (56) | 38 (70) | 0.099 |
| Duration, days | 8 ± 8 | 7 ± 8 | 0.710 |
| Fluoroquinolone use | 22 (36) | 24 (44) | 0.336 |
| Duration, days | 6 ± 6 | 5 ± 4 | 0.913 |
| Extended-spectrum cephalosporin use | 3 (5) | 16 (29) | 0.001b |
| Duration, days | 1 ± 3 | 2 ± 5 | 0.002 |
| Carbapenem use | 5 (8) | 7 (12) | 0.306 |
| Duration, days | 3 ± 3 | 4 ± 1 | 0.428 |
| Aminoglycoside use | 10 (16) | 15 (27) | 0.233 |
| Duration, days | 2 ± 5 | 2 ± 4 | 0.154 |
| Metronidazole use | 4 (7) | 3 (6) | 0.559 |
| Duration, days | 0.4 ± 2 | 0.3 ± 2 | 0.785 |
Data are presented as mean ± standard deviation or number (percentage). Odds ratio (95% confidence interval) = a3.8 (1.5 to 10) and b7.3 (1.9 to 26.9).
Discussion
Our results suggest that S. maltophilia colonisation and/or infection is not common in this population of immunocompetent ICU patients. COPD and duration of antibiotic treatment are independently associated with ICU-acquired S. maltophilia. ICU-acquired S. maltophilia is associated with high ICU mortality and morbidity rates. In addition, ICU-acquired infection related to S. maltophilia is an independent risk factor for ICU mortality.
In the SENTRY Antimicrobial Surveillance Program, S. maltophilia represented 0.6% to 0.9% of all isolates collected during a three year period [7]. In that study, the respiratory tract was the most commonly reported S. maltophilia site of infection in all geographic regions. Incidence of ICU-acquired S. maltophilia found by our study (2%) is higher than previously reported. This is probably related to the fact that screening of patients with S. maltophilia was not performed in previous studies. Our study differs from previous studies in its methodology. In previous studies, patients with infection related to S. maltophilia were compared with patients without S. maltophilia infection. However, in our study immunocompetent patients with ICU-acquired S. maltophilia were compared with immunocompetent patients without NF-GNB colonisation and/or infection. Risk factors for S. maltophilia and other NF-GNB are probably similar. Therefore, exclusion of patients with colonisation and/or infection related to NF-GNB other than S. maltophilia probably allowed a more accurate evaluation of risk factors for ICU-acquired S. maltophilia.
COPD was identified as an independent risk factor for ICU-acquired S. maltophilia. Previous studies identified COPD as a risk factor for VAP and for respiratory tract colonisation by GNB [20,21]. Factors predisposing to respiratory tract colonisation include impairment of mucosal clearance and loss of mucosal integrity [22]. A multicentre study evaluated the relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbation of COPD hospitalised in pneumology units [23]. P. aeruginosa was isolated more frequently in patients with low FEV1 (forced expiratory volume in one second). Unfortunately, information on severity of COPD was not available in our study. Another potential explanation for the association between COPD and ICU-acquired S. maltophilia is the frequent antibiotic use in patients with COPD. Patients with severe COPD are prone to frequent acute exacerbations requiring antimicrobial therapy [24]. In our study, rate of prior antibiotic use was higher in patients with COPD than in patients without COPD. However, this difference did not reach statistical significance.
Prolonged duration of antibiotic treatment is a well-known risk factor for emergence of MDR bacteria [25,26]. Shortening duration of antibiotic treatment could be useful in preventing ICU-acquired S. maltophilia. Two recent randomised trials demonstrated that shorter duration of antibiotic treatment is effective and safe in ICU patients with VAP [26,27]. In addition, serial measurements of clinical pulmonary infection score can define the clinical course of VAP resolution [28]. Based on this score, identifying patients with good outcome as early as day three could be of help to define strategies to shorten the duration of antibiotic treatment.
Recent reports documented an increasing incidence of NF-GNB, including S. maltophilia, in patients with cystic fibrosis [29,30]. In our study, cystic fibrosis was not significantly associated with S. maltophilia acquisition. However, few patients with cystic fibrosis were included in this study. Marchac et al. [31] reported that prevalence of S. maltophilia, in respiratory tract of patients with cystic fibrosis, increased from 3.3% to 15% during the last decade. Antibiotic treatment, isolation of Aspergillus fumigatus in sputum, and oral corticosteroid use were significantly associated with S. maltophilia [31]. Another recent study found no association between lung function decline and S. maltophilia acquisition in patients with cystic fibrosis, suggesting that S. maltophilia is rather a marker of worse lung function in these patients [32].
Carbapenem use was not significantly associated with ICU-acquired S. maltophilia. However, previous studies identified carbapenem use as an independent risk factor for S. maltophilia acquisition [33,34]. These different results could be explained by the small number of patients treated with carbapenem in our study. However, other studies found no relationship between carbapenem use and S. maltophilia acquisition, whereas agents other than carbapenems were identified as risk factors for the acquisition of S. maltophilia [35,36]. These findings suggest that exposure to broad-spectrum antibiotics might be more important than exposure to any single agent. Immunosuppression is another well-known risk factor for S. maltophilia acquisition [14,37]. Although patients with severe immunosuppression were excluded from our study, ICU patients may be at risk for immunoparalysis. A temporary immunoparalysis may occur in critically ill patients, resulting in higher risk for nosocomial infections [38].
Although risk factors for colonisation and infection related to S. maltophilia were similar, outcomes of patients with colonisation or infection related to S. maltophilia were clearly different. A recent meta-analysis demonstrated that risk factors for colonisation and infection related to MDR bacteria were similar [39].
In our study, S. maltophilia was associated with significantly increased ICU mortality and longer duration of mechanical ventilation and ICU stay. However, S. maltophilia was previously considered to have limited pathogenicity. In a recent retrospective study, S. matophilia was isolated in the respiratory tract specimens of 64 patients without pneumonia [11]. No significant difference was found in mortality rate between treated and untreated patients. However, few patients (29%) were mechanically ventilated. In another retrospective study, patients with S. maltophilia VAP were compared with patients with late-onset VAP related to other GNB [16]. Although mortality rate was similar in patients with S. maltophilia VAP and patients with late-onset VAP related to other GNB, S. maltophilia was associated with increased patient morbidity. Recent studies indicated that infection with this organism was associated with significant morbidity and mortality, particularly in severely compromised patients [12,13,15]. A retrospective case study, including 69 patients with infection or colonisation related to multi-resistant S. maltophilia, has evaluated risk factors for mortality [40]. McCabe score and organ dysfunction were associated with increased risk for mortality in these patients. These results suggest that infection related to S. maltophilia has an indirect impact on mortality. In contrast, our results suggest a direct impact of S. maltophilia infection on ICU mortality given that this infection was found to be an independent risk factor for ICU mortality. In cases with ICU-acquired infection related to S. maltophilia, inappropriate initial antibiotic treatment was associated with increased ICU mortality rate. Inappropriate initial antibiotic treatment is a well-known risk factor for mortality in patients with infection related to NF-GNB [5,6].
Our study has several limitations. First, it was performed in a single ICU. Therefore, our results may not be applicable to other ICU patients. Similarly, only immunocompetent patients were included. This precludes application of our results to patients with immunosuppression. Second, during the study period, no molecular typing was performed on S. maltophilia isolates. Therefore, the impact of patient-to-patient transmission could not be determined. Previous studies using molecular typing in newborn and pediatric ICUs have demonstrated that cross-transmission of S. maltophilia could be identified using molecular typing [41,42]. However, no S. maltophilia outbreak occurred during the study period. Finally, the number of patients with ICU-acquired S. maltophilia was small. Therefore, some of the trends observed in this study could have reached statistical significance if the study sample had been larger.
Conclusion
S. maltophilia colonisation and/or infection is not frequent in this cohort of immunocompetent ICU patients. COPD and duration of antibiotic treatment are independently associated with ICU-acquired S. maltophilia. ICU-acquired S. maltophilia is associated with high mortality and morbidity rates. In addition, ICU-acquired infection related to S. maltophilia is an independent risk factor for ICU mortality.
Key messages
• ICU-acquired S. maltophilia is not common among this cohort of immunocompetent ICU patients.
• COPD and duration of antibiotic treatment are independently associated with ICU-acquired S. maltophilia and with ICU-acquired infection related to S. maltophilia.
• ICU-acquired S. maltophilia is associated with high mortality and morbidity rates. In addition, ICU-acquired infection related to S. maltophilia is an independent risk factor for ICU mortality.
Abbreviations
CI = confidence interval; COPD = chronic obstructive pulmonary disease; ICU = intensive care unit; MDR = multi-drug-resistant; NF-GNB = non-fermenting Gram-negative bacilli; OR = odds ratio; SAPS = Simplified Acute Physiology Score; VAP = ventilator-associated pneumonia.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SN and AD designed the study. All authors contributed to data collection. CDP performed statistical analyses. SN wrote the manuscript, all authors participated in its critical revision. SN had full access to all data in the study and had final response ability for the decision to submit for publication. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
This work was presented in part at the 101st American Thoracic Society conference, May 20–25, 2005, San Diego, CA, USA.
References
- Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR, Payen D. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–353. doi: 10.1097/01.CCM.0000194725.48928.3A. [DOI] [PubMed] [Google Scholar]
- Aloush V, Navon-Venezia S, Seigman-Igra Y, Cabili S, Carmeli Y. Multidrug-resistant Pseudomonas aeruginosa: risk factors and clinical impact. Antimicrob Agents Chemother. 2006;50:43–48. doi: 10.1128/AAC.50.1.43-48.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthelot P, Grattard F, Mallaval FO, Ros A, Lucht F, Pozzetto B. Epidemiology of nosocomial infections due to Pseudomonas aeruginosa, Burkholderia cepacia and Stenotrophomonas maltophilia. Pathol Biol. 2005;53:341–348. doi: 10.1016/j.patbio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Nseir S, Ader F, Marquette CH, Durocher A. Impact of fluoroquinolone use on multidrug-resistant bacteria emergence. Pathol Biol. 2005;53:470–475. doi: 10.1016/j.patbio.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob Agents Chemother. 2005;49:1306–1311. doi: 10.1128/AAC.49.4.1306-1311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Kim EC, Oh MD, Choe KW. Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother. 2005;49:760–766. doi: 10.1128/AAC.49.2.760-766.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gales AC, Jones RN, Forward KR, Linares J, Sader HS, Verhoef J. Emerging importance of multidrug-resistant Acinetobacter species and Stenotrophomonas maltophilia as pathogens in seriously ill patients: geographic patterns, epidemiological features, and trends in the SENTRY Antimicrobial Surveillance Program (1997–1999) Clin Infect Dis. 2001;32(Suppl 2):S104–S113. doi: 10.1086/320183. [DOI] [PubMed] [Google Scholar]
- Senol E. Stenotrophomonas maltophilia: the significance and role as a nosocomial pathogen. J Hosp Infect. 2004;57:1–7. doi: 10.1016/j.jhin.2004.01.033. [DOI] [PubMed] [Google Scholar]
- Ferrara AM. Potentially multidrug-resistant non-fermentative Gram-negative pathogens causing nosocomial pneumonia. Int J Antimicrob Agents. 2006;27:183–195. doi: 10.1016/j.ijantimicag.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Looney WJ. Role of Stenotrophomonas maltophilia in hospital-acquired infection. Br J Biomed Sci. 2005;62:145–154. doi: 10.1080/09674845.2005.11732702. [DOI] [PubMed] [Google Scholar]
- Pathmanathan A, Waterer GW. Significance of positive Stenotrophomonas maltophilia culture in acute respiratory tract infection. Eur Respir J. 2005;25:911–914. doi: 10.1183/09031936.05.00096704. [DOI] [PubMed] [Google Scholar]
- Tsai WP, Chen CL, Ko WC, Pan SC. Stenotrophomonas maltophilia bacteremia in burn patients. Burns. 2006;32:155–158. doi: 10.1016/j.burns.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Friedman ND, Korman TM, Fairley CK, Franklin JC, Spelman DW. Bacteraemia due to Stenotrophomonas maltophilia: an analysis of 45 episodes. J Infect. 2002;45:47–53. doi: 10.1053/jinf.2002.0978. [DOI] [PubMed] [Google Scholar]
- Lai CH, Chi CY, Chen HP, Chen TL, Lai CJ, Fung CP, Yu KW, Wong WW, Liu CY. Clinical characteristics and prognostic factors of patients with Stenotrophomonas maltophilia bacteremia. J Microbiol Immunol Infect. 2004;37:350–358. [PubMed] [Google Scholar]
- Wang WS, Liu CP, Lee CM, Huang FY. Stenotrophomonas maltophilia bacteremia in adults: four years' experience in a medical center in northern Taiwan. J Microbiol Immunol Infect. 2004;37:359–365. [PubMed] [Google Scholar]
- Hanes SD, Demirkan K, Tolley E, Boucher BA, Croce MA, Wood GC, Fabian TC. Risk factors for late-onset nosocomial pneumonia caused by Stenotrophomonas maltophilia in critically ill trauma patients. Clin Infect Dis. 2002;35:228–235. doi: 10.1086/341022. [DOI] [PubMed] [Google Scholar]
- Nseir S, Di Pompeo C, Soubrier S, Lenci H, Delour P, Onimus T, Saulnier F, Mathieu D, Durocher A. Effect of ventilator-associated tracheobronchitis on outcome in patients without chronic respiratory failure: a case-control study. Crit Care. 2005;9:R238–R245. doi: 10.1186/cc3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:S77–121. [PubMed] [Google Scholar]
- Torres A, Aznar R, Gatell JM, Jimenez P, Gonzalez J, Ferrer A, Celis R, Rodriguez-Roisin R. Incidence, risk, and prognosis factors of nosocomial pneumonia in mechanically ventilated patients. Am Rev Respir Dis. 1990;142:523–528. doi: 10.1164/ajrccm/142.3.523. [DOI] [PubMed] [Google Scholar]
- Nseir S, Di Pompeo C, Soubrier S, Cavestri B, Jozefowicz E, Saulnier F, Durocher A. Impact of ventilator-associated pneumonia on outcome in patients with COPD. Chest. 2005;128:1650–1656. doi: 10.1378/chest.128.3.1650. [DOI] [PubMed] [Google Scholar]
- Reynolds HY. Bacterial adherence to respiratory tract mucosa – a dynamic interaction leading to colonization. Semin Respir Infect. 1987;2:8–19. [PubMed] [Google Scholar]
- Miravitlles M, Espinosa C, Fernandez-Laso E, Martos JA, Maldonado JA, Gallego M. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Study Group of Bacterial Infection in COPD. Chest. 1999;116:40–46. doi: 10.1378/chest.116.1.40. [DOI] [PubMed] [Google Scholar]
- Wilson R. Treatment of COPD exacerbations: antibiotics. Eur Respir Rev. 2005;14:32–38. [Google Scholar]
- Nseir S, Di Pompeo C, Soubrier S, Delour P, Lenci H, Roussel-Delvallez M, Onimus T, Saulnier F, Mathieu D, Durocher A. First-generation fluoroquinolone use and subsequent emergence of multiple drug-resistant bacteria in the intensive care unit. Crit Care Med. 2005;33:283–289. doi: 10.1097/01.CCM.0000152230.53473.A1. [DOI] [PubMed] [Google Scholar]
- Chastre J, Wolff M, Fagon JY, Chevret S, Thomas F, Wermert D, Clementi E, Gonzalez J, Jusserand D, Asfar P, Perrin D, Fieux F, Aubas S. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290:2588–2598. doi: 10.1001/jama.290.19.2588. [DOI] [PubMed] [Google Scholar]
- Micek ST, Ward S, Fraser VJ, Kollef MH. A randomized controlled trial of an antibiotic discontinuation policy for clinically suspected ventilator-associated pneumonia. Chest. 2004;125:1791–1799. doi: 10.1378/chest.125.5.1791. [DOI] [PubMed] [Google Scholar]
- Luna CM, Blanzaco D, Niederman MS, Matarucco W, Baredes NC, Desmery P, Palizas F, Menga G, Rios F, Apezteguia C. Resolution of ventilator-associated pneumonia: prospective evaluation of the clinical pulmonary infection score as an early clinical predictor of outcome. Crit Care Med. 2003;31:676–682. doi: 10.1097/01.CCM.0000055380.86458.1E. [DOI] [PubMed] [Google Scholar]
- Lambiase A, Raia V, Del Pezzo M, Sepe A, Carnovale V, Rossano F. Microbiology of airway disease in a cohort of patients with cystic fibrosis. BMC Infect Dis. 2006;6:4. doi: 10.1186/1471-2334-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkamp G, Wiedemann B, Rietschel E, Krahl A, Gielen J, Barmeier H, Ratjen F. Prospective evaluation of emerging bacteria in cystic fibrosis. J Cyst Fibros. 2005;4:41–48. doi: 10.1016/j.jcf.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Marchac V, Equi A, Bihan-Benjamin C, Hodson M, Bush A. Case-control study of Stenotrophomonas maltophilia acquisition in cystic fibrosis patients. Eur Respir J. 2004;23:98–102. doi: 10.1183/09031936.03.00007203. [DOI] [PubMed] [Google Scholar]
- Goss CH, Mayer-Hamblett N, Aitken ML, Rubenfeld GD, Ramsey BW. Association between Stenotrophomonas maltophilia and lung function in cystic fibrosis. Thorax. 2004;59:955–959. doi: 10.1136/thx.2003.017707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarino ME, Stevens LE, Schable B, Mayers G, Miller JM, Burke JP, Jarvis WR. Risk factors for epidemic Xanthomonas maltophilia infection/colonization in intensive care unit patients. Infect Control Hosp Epidemiol. 1992;13:201–206. doi: 10.1086/646510. [DOI] [PubMed] [Google Scholar]
- Sanyal SC, Mokaddas EM. The increase in carbapenem use and emergence of Stenotrophomonas maltophilia as an important nosocomial pathogen. J Chemother. 1999;11:28–33. doi: 10.1179/joc.1999.11.1.28. [DOI] [PubMed] [Google Scholar]
- Senol E, DesJardin J, Stark PC, Barefoot L, Snydman DR. Attributable mortality of Stenotrophomonas maltophilia bacteremia. Clin Infect Dis. 2002;34:1653–1656. doi: 10.1086/340707. [DOI] [PubMed] [Google Scholar]
- VanCouwenberghe CJ, Farver TB, Cohen SH. Risk factorsassociated with isolation of Stenotrophomonas (Xanthomonas) maltophilia in clinical specimens. Infect Control Hosp Epidemiol. 1997;18:316–321. doi: 10.1086/647618. [DOI] [PubMed] [Google Scholar]
- Rolston KV, Kontoyiannis DP, Yadegarynia D, Raad II. Nonfermentative gram-negative bacilli in cancer patients: increasing frequency of infection and antimicrobial susceptibility of clinical isolates to fluoroquinolones. Diagn Microbiol Infect Dis. 2005;51:215–218. doi: 10.1016/j.diagmicrobio.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Wunderink RG. Nosocomial pneumonia, including ventilator-associated pneumonia. Proc Am Thorac Soc. 2005;2:440–444. doi: 10.1513/pats.2005080-83JS. [DOI] [PubMed] [Google Scholar]
- Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, enterococcus, gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med. 2002;136:834–844. doi: 10.7326/0003-4819-136-11-200206040-00013. [DOI] [PubMed] [Google Scholar]
- Tsiodras S, Pittet D, Carmeli Y, Eliopoulos G, Boucher H, Harbarth S. Clinical implications of Stenotrophomonas maltophilia resistant to trimethoprim-sulfamethoxazole: a study of 69 patients at 2 university hospitals. Scand J Infect Dis. 2000;32:651–656. doi: 10.1080/003655400459577. [DOI] [PubMed] [Google Scholar]
- Gulcan H, Kuzucu C, Durmaz R. Nosocomial Stenotrophomonas maltophilia cross-infection: three cases in newborns. Am J Infect Control. 2004;32:365–368. doi: 10.1016/j.ajic.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Lanotte P, Cantagrel S, Mereghetti L, Marchand S, Van der MN, Besnier JM, Laugier J, Quentin R. Spread of Stenotrophomonas maltophilia colonization in a pediatric intensive care unit detected by monitoring tracheal bacterial carriage and molecular typing. Clin Microbiol Infect. 2003;9:1142–1147. doi: 10.1046/j.1469-0691.2003.00785.x. [DOI] [PubMed] [Google Scholar]


