Abstract
Endotoxin tolerance is defined as a reduced responsiveness to a lipopolysaccharide (LPS) challenge following a first encounter with endotoxin. Endotoxin tolerance protects against a lethal challenge of LPS and prevents infection and ischemia-reperfusion damage. Endotoxin tolerance is paralleled by a dramatic reduction of tumor necrosis factor (TNF) production and some other cytokines in response to LPS. Endotoxin tolerance involves the participation of macrophages and mediators, such as glucocorticoids, prostaglandins, IL-10, and transforming growth factor-β. Endotoxin tolerance is accompanied by the up-regulation of inhibitory molecules that down-regulate the Toll-like receptor (TLR)4-dependent signaling pathway. Cross-tolerance between LPS and other TLR specific ligands, as well as IL-1 and TNF, has been regularly reported. A similar loss of LPS reactivity has been repeatedly reported in circulating leukocytes of septic patients and in patients with non-infectious systemic inflammation response syndrome (SIRS). Studies on cellular signaling within leukocytes from septic and SIRS patients reveal numerous alterations reminiscent of those observed in endotoxin tolerant cells. However, altered responsiveness to LPS of leukocytes from sepsis and SIRS patients is not synonymous with a global down-regulation of cellular reactivity. The term 'cellular reprogramming', which has been proposed to qualify the process of endotoxin tolerance, defines well the immune status of circulating leukocytes in septic and SIRS patients.
In vivo endotoxin tolerance
Paul Beeson first reported endotoxin tolerance in 1946 [1] as the abolition of the fever response of rabbits undergoing repeated daily injection of the same dose of typhoid vaccine. Later, it was shown that plasma of a tolerant rabbit could passively transfer tolerance to pyrogenicity of bacterial endotoxin to another animal [2]. In human volunteers, it was shown that inoculation of live Salmonella typhosa led to a reduced fever in response to endotoxin or killed bacteria compared to before infection [3]. Interestingly, a similar observation was reported with volunteers inoculated with Plasmodium cynomolgi by mosquito bites [4], suggesting that cross-tolerization between different stimuli could occur. In addition to human volunteers, reduced fever to endotoxin or killed bacteria was reported in different infections, such as in patients with pyelonephritis [5], in patients convalescent from typhoid and paratyphoid fever [6], and in patients recovering from malaria [7].
Not only can a pretreatment with endotoxin reduce subsequent lipopolysaccharide (LPS)-induced fever in rabbits, it also prevents LPS-induced lethality [8]. In mice, even when LPS-induced lethality was dramatically enhanced by galactosamine treatment, LPS tolerization could prevent mortality [9]. By specific cell transfer from LPS-resistant to LPS-sensitive mice, Freudenberg and Galanos [9] elegantly demonstrated the role of macrophages in the induction of endotoxin tolerance in vivo.
Endotoxin tolerance and cytokine production
Tumor necrosis factor (TNF) is most probably the best marker of endotoxin tolerance as assessed by its dramatically reduced production following an LPS challenge in tolerized animals, in contrast to its sharp and fast peak in response to a first injection of LPS [10]. Interestingly, not all cytokines behave similarly. In mouse models of endotoxin tolerance, IL-6 and IFN-γ released in the circulation following an LPS challenge are also dramatically blunted, whereas this is not the case for IL-12p70, and the chemokines KC and MCP-1, the circulating levels of which were reduced but not as much as TNF and the previously mentioned cytokines [11]. In contrast, levels of IL-1β and IL-18 were maintained independently of the tolerization process. In humans, induction of tolerance by monophosphoryl lipid A led to a reduction of circulating TNF, IL-6 and IL-8 in response to a subsequent LPS challenge [12].
In vitro pre-treatment of macrophage cell lines with LPS, as well as freshly isolated macrophages and monocytes, rendered the cells hyporeactive to a second LPS stimulus, particularly in terms of cytokine production. In vitro tolerization of human monocytes could be partially mimicked by IL-1, IL-10 or TGFβ pre-treatment [13], and the use of anti-IL-10 or anti-TGFβ antibodies during the step of tolerization could prevent the phenomenon [14]. Although IL-10 contributes to endotoxin tolerance, its presence is dispensable as in vivo tolerization could be achieved in IL-10 deficient mice [15].
Endotoxin tolerance and resistance to inflammatory process or infection
It is worth recalling that endotoxin tolerance has been associated with increased resistance and protection against tissue injuries and even mortality in models such as infected thermal injury [16], hepatic ischemia/reperfusion [17], renal ischemia/reperfusion [18], coronary occlusion [19], or hemorrhagic shock [20]. All these increased resistances to stressful situations further illustrate that endotoxin tolerance is far more than a single altered inflammatory responsiveness to LPS.
In contrast, some studies suggested that the induction of endotoxin tolerance might impair resistance to infectious processes. For example, a reduced level of IFN was reported in animals pretreated with LPS and further challenged with Newcastle disease virus [21]. Moreover, a reduced leishmanicidal activity was observed after pre-exposure of macrophages to LPS [22], and an impaired lung clearance of Pseudomonas aeruginosa was shown after LPS exposure [23]. Recent investigation with other infectious models using either Cryptococcus neoformans [24] or Salmonella enteritica [25] established that LPS tolerant mice had an increased resistance to fungal or bacterial infection, associated with a reduced burden of pathogens within the tissues. Based upon experiments combining pentoxifylline and LPS pretreatment [24], one can postulate that the boost of TNF, among other cytokines, during the first stimulation by LPS, later increased the resistance of the animals to the infectious process. This is in agreement with the protective role of inflammatory cytokines regularly reported since the 1980s. Accordingly, it is difficult to assume that endotoxin tolerance per se is directly linked to the increased susceptibility of systemic inflammation response syndrome (SIRS) patients to nosocomial infections. Indeed, leukocytes from sepsis and SIRS patients also display altered antigen presentation, direct microbicidal activity, and oxidative burst.
Leukocyte reprogramming in sepsis and systemic inflammatory response syndrome
An altered responsiveness of circulating human monocytes has been regularly reported in sepsis patients. IL-1α, IL-1β, IL-6, and TNF production upon ex vivo activation of patients' monocytes were significantly reduced in response to LPS [26]. Normal reactivity was never restored in non-surviving patients in contrast to those who recovered. This altered ex vivo cytokine production was reproduced in human volunteers receiving an injection of LPS [27].
Altered ex vivo cytokine production is not a generalized phenomenon
The reduction of the capacity of circulating leukocytes to produce cytokine depends on several parameters. The change of monocyte reactivity is a reflection of subtle modifications that differ depending upon the nature of the stress. For example, in patients undergoing major surgery, two days post-surgery, the ex vivo production of TNF in response to LPS was never reported to be reduced [28]. In contrast, trauma patients displayed a long lasting hypo-reactivity several days after their admission [29]. It is possible that the use of anesthetic drugs during surgery may limit the cell reprogramming following surgical injury, in contrast to what happens in patients after trauma or burn. If so, it would imply that neuromediators contribute to control cellular reprogramming.
Many experiments assessing leukocyte hyporeactivity were performed in whole blood assays, and are difficult to interpret. First, some cytokines can be produced both by mononuclear and by polymorphonuclear cells present in whole blood samples (for example, IL-1ra), and the responsiveness of each individual population may be differently affected by the insult. This may explain why, in sepsis, the analysis of LPS-induced IL-1ra performed in whole blood displayed an enhanced release compared to healthy controls [30] while that produced by isolated neutrophils was reduced [31]. Second, the presence of immunosuppressive factors within the plasma may play an inhibitory role during the ex vivo culture and may mask or influence the individual behavior of leukocytes, once isolated [32,33]. For example, in sepsis, ex vivo induction of TNF, IL-6 and IL-10 by heat-killed Escherichia coli was shown to be reduced in whole blood compared to healthy controls, whereas no difference was noticed with isolated monocytes [34,35]. Despite this possibility, it is worth mentioning that, in trauma patients, numerous whole blood cultures revealed an enhanced release of cytokines in response to different agonists.
Monocytes from septic patients have a diminished capacity to release TNFα, IL-1α, IL-1β, IL-6, and IL-12 [30,36], whereas this is not the case for IL-1ra [30], granulocyte colony stimulatign factor [37] and macrophage migration inhibitory factor [38]. We recently observed an enhanced production of IL-10 by monocytes from septic patients in response to both LPS and Pam3CysSK4 [35]. An enhanced or unchanged IL-10 production was observed with circulating leukocytes of patients after surgery [39], trauma [29], and in resuscitated patients after cardiac arrest (RCA) [40]. In sepsis there are controversial reports [41,42]. The fact that, after LPS-triggering, monocytes can display a reduced production of TNF and an unaltered or even enhanced production of IL-10, illustrates that monocytes can still sense LPS, but that the intracellular signaling has been modified to limit the production of pro-inflammatory cytokines and to maintain or to favor that of anti-inflammatory ones.
There are still few studies that have investigated the responsiveness of circulating leukocytes of SIRS patients to agonists other than LPS. Recently, we showed in sepsis a significantly reduced reactivity of monocytes to Pam3CysSK4, a specific synthetic Toll-like receptor (TLR)2 agonist, compared to healthy controls. In contrast, the production of TNF induced by a TLR2 ligand was similar to that of healthy controls in RCA and trauma patients [35]. There are still too few studies with specific TLR9 ligands, and with IL-1 and TNF as stimulating agents [29] to conceive a general idea of the nature of the responsiveness to these specific activators.
Cross-tolerance has been regularly reported in experimental models of endotoxin tolerance between LPS and other TLR ligands. However, recent investigations suggest that the specificity of TLR agonists may influence the observation of cross-tolerance, since highly purified LPS (TLR4 ligand) did not tolerize macrophages to Pam3CysSK4 (TLR2 ligand) [43]. Revisiting the concept of cross-tolerance with Gram-positive bacteria in a mouse model of endotoxin tolerance, we showed that the phenomenon was only transient, especially in the blood compartment [44]. Although the use of highly specific TLR agonists is useful to further understand the alteration of specific signaling pathways within cells from SIRS patients, the response to whole bacteria may represent a more relevant and physiological approach to monitor the immune status. Many reports showed that leukocytes from SIRS and sepsis patients remained responsive to whole bacteria. Cells from SIRS patients normally responded to heat-killed Staphylococcus aureus and Streptococcus pyogenes [29]. These observations are in agreement with previous reports in sepsis performed with P. aeruginosa [41] and Salmonella typhimurium [45], and others in non-infectious SIRS with S. aureus [29,40,46]; they differ from other observations that showed in sepsis a reduced TNF production in response to S. aureus and E. coli [34,47].
Mechanisms of leukocyte reprogramming
Desensitizing agents in plasma
The presence of deactivating or immunosuppressive agents within the blood stream may contribute to the hyporeactivity of circulating leukocytes. In the late 1970s, it was reported that sera of burn patients were able to suppress the proliferative response of normal cells [48]. Prins and colleagues [49] showed that sera from septic patients had the capacity to down-regulate the TNF production by activated monocytes from healthy donors, The fact that 'septic plasma' behaves as an immunosuppressive milieu [32] is illustrated in human volunteers by the capacity of endotoxin to induce plasma inhibitors [50]. Most interestingly, in septic patients, this suppressive effect was significantly reduced after passage of plasma through a resin and after incubation with anti-IL-10 antibodies [51]. IL-10 was identified as a major functional deactivator of monocytes in human septic shock plasma [52]. However, we demonstrated that IL-10 was not sufficient to explain the observed dysregulation that occurs in septic patients [53] and IL-10 knock out mice can still be tolerized to endotoxin [15]. TGFβ was also shown in animal models of hemorrhagic shock or sepsis to be the causative agent of the depressed splenocyte responsiveness [54]. Monocytes from immunocompromized trauma patients seem to be a source of TGFβ [55], and TGFβ released by apoptotic T cells contributes to this immunosuppressive milieu [56]. In addition, there is accumulating evidence for a strong interaction between components of the nervous and the immune systems. Numerous neuromediators have been shown to behave as immunosuppressors. Catecholamines, found to be at higher concentrations in stressful situations [57], suppress the activity of immuno-competent cells, inhibit TNF production and favor IL-10 release. Alpha-melanocyte-stimulating hormone also contributes to immunosuppression by inducing IL-10 production by human monocytes [58]. In addition, vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide directly inhibit endotoxin induced pro-inflammatory cytokine secretion [59]. SIRS is also associated with an activation of the hypothalamus-pituitary-adrenal axis, which leads to the release of glucocorticoids, well known for their potent ability to limit cytokine production [60]. Prostaglandins are produced during sepsis and can also contribute to the down regulation of cytokine production [61]. Finally, the levels of circulating heat shock proteins are elevated in SIRS and sepsis patients [62]. Since it has been shown that over-expression of heat shock proteins can inhibit LPS induced production of cytokines [63], one can postulate that they may play a role in desensitizing circulating cells.
Endotoxin neutralizing molecules in plasma
When the cells from healthy controls were incubated with plasma from non-infectious SIRS patients, a strong inhibition of LPS-induced TNF production was observed, suggesting that patients' plasma contains inhibitory mediators [64]. In contrast, SIRS plasma did not significantly affect the heat-killed S. aureus-induced TNF production. SIRS plasma, which contains high levels of sCD14 [65] and high levels of LPS-binding protein [66], may be particularly inhibitory towards the LPS-induced activation because of the presence of these molecules, high concentrations of which are known to specifically inhibit LPS. In addition, plasma lipoproteins are also known to neutralize endotoxins [67], and Warren and colleagues [68] have shown that LPS bound much more rapidly to lipoprotein fractions in tolerant serum than in normal serum. More recently, enhanced levels of ubiquitin, an 8.6 kDa protein involved in intracellular function, have been found in serum of sepsis and trauma patients and shown to specifically inhibit TNF induction by LPS [69]. Altogether, these observations may partially explain why LPS responsiveness is specifically reduced in SIRS compared to other activators.
Nuclear factor-kappa B inhibition
Nuclear factor-kappa B (NF-κB) is critical for maximal expression of many cytokines involved in the pathogenesis of inflammation. Activation and regulation of NF-κB are tightly controlled by a group of inhibitory proteins (IκB), which maintain NF-κB in the cytoplasm of effector cells. Blackwell and co-workers [70] investigated the role of NF-κB in the mechanism of endotoxin tolerance in a rat alveolar macrophage cell line. Tolerance, monitored by cytokine production, was associated with an impaired activation of NF-κB and a depletion of both p65 and p50 forms. This study suggested that endotoxin tolerance may be mediated by limiting the amount of NF-κB available for activation and, thus, inhibiting transcription of NF-κB-dependent genes. On the other hand, Ziegler-Heitbrock and colleagues [71] demonstrated that endotoxin tolerance in a monocytic cell line was associated with an increase of the inactive p50 homodimer of NF-κB and a decrease of the p50p65 active heterodimer. We undertook a study on NF-κB expression in mononuclear cells of patients with severe sepsis or major trauma. Subsequent to an in vitro stimulation of peripheral blood mononuclear cells (PBMCs) with LPS, the expression of both p65p50 and p50p50 was low in survivors of sepsis, while non-survivors of sepsis showed a predominance of the inactive homodimer and a low p65p50/p50p50 ratio when compared to controls [72]. In the latter group of patients there was a reverse correlation between plasma IL-10 levels and the p65p50/p50p50 ratio after in vitro LPS stimulation. The reduced expression of nuclear NF-κB was not due to its inhibition by IκBα since very low expression of IκBα and low levels of p65 and p50 were found in the cytoplasm of PBMCs from sepsis patients when compared to controls. These results demonstrate that, upon LPS activation, PBMCs of SIRS patients show patterns of NF-κB expression that resemble those reported during LPS tolerance: a global down-regulation of NF-κB in survivors of sepsis, or the presence of large amounts of the inactive homodimer in the non-survivors. In trauma patients, we observed a long-term reduction of both p65/p50 heterodimers and p50/p50 homodimers and a reduced p65p50/p50p50 ratio after LPS stimulation in vitro [73].
Down regulation of TLR-associated signaling pathways
Still very few studies have addressed in humans the link between the altered responsiveness to LPS of monocytes of sepsis and SIRS patients and the modification of the TLR4 signaling pathways. In the past few years, numerous intracellular molecules that negatively regulate LPS-activated signaling pathways have been discovered (Figure 1). IL-1 receptor associated kinase (IRAK)-M prevents the dissociation of IRAK-1 and IRAK-4 from myeloid differentiation (MyD)88 and the formation of IRAK-TNF receptor associated factor (TRAF)6 complexes, and is a negative regulator of TLR signaling. Interestingly, endotoxin tolerance is significantly reduced in IRAK-M deficient mice [74]. It has been recently reported that monocytes from septic patients, when stimulated with LPS ex vivo, express IRAK-M mRNA more rapidly than cells from healthy donors [75]. Phosphatidylinositol 3-kinase behaves as an early inhibitor of TLR signaling [76]. Learn and colleagues [77] reported in septic patients that the repressed production of IL-1β and the selective elevation of the secreted form of IL-1Ra in response to LPS were linked to a probably altered IRAK-dependent signaling pathway and a maintained efficient phosphatidylinositol 3-kinase-dependent signaling pathway. Toll interacting protein (Tollip), an adapter protein found to associate with the cytoplasmic TIR domain of IL-1R, TLR2 and TLR4, potently suppresses the activity of IRAK after TLR activation [78]. A splice variant of MyD88, termed MyD88 short (MyD88s), induced upon LPS activation, is defective in its ability to induce IRAK phosphorylation and behaves as dominant-negative inhibitor [79]. Single immunoglobulin IL-1R-related molecule (SIGIRR), a member of the TLR/IL-1R superfamily, is a negative modulator of the signaling induced by IL-1 or TLR4 ligands in other cells than macrophages [80]. Suppressor of cytokine signaling (SOCS)-1, promptly induced in macrophages upon LPS stimulation, is a negative regulator molecule of the JAK-STAT signal cascade. Interestingly, endotoxin tolerance cannot be observed in mice deficient for SOCS-1 [81]. We recently investigated the Tollip, SOCS1, MyD88s and SIGIRR regulators in sepsis and RCA patients. In monocytes of sepsis patients, we found that the mRNA expression of Tollip and SOCS1 was unchanged, while that of MyD88s and SIGIRR was significantly enhanced compared to healthy controls [35]. Other molecules involved in down-regulating the TLR4-induced MyD88-dependent signaling pathway would be worth investigating. In addition to SIGIRR, two other surface receptors are known to down regulate TLR4-signaling, namely RP105, which prevents TLR4/MD2 activation [82], and ST2, which interacts with and inhibits MyD88 and Mal/TIRAP [83]. Intracellular inhibitory molecules include: Triad3A, which favors ubiquitynilation of TLR4 and promotes its degradation via the proteosome [84]; Flightless I homolog, which interacts with MyD88 and interferes with the formation of the TLR4-MyD88 complex [85]; Monarch-1, which associates with IRAK-1, resulting in the blockage of IRAK-1 hyperphosphorylation [86]; A20, which removes ubiquitin moieties from the signaling molecule TRAF6 [87]; SHIP, which is produced in response to TGFβ exerting its inhibitory effects, primarily through the hydrolysis of the phosphatidylinositol 3-kinase [88]; TRAF4, which interacts and counteracts TRAF6 and TRIF molecules [89]; FLN29, which contains a TRAF-6-related zinc finger motif suppressing NF-κB and mitogen-activated protein kinase activation downstream of TRAF6 [90]; ABIN-3, which inhibits NF-κB activation, downstream of TRAF6, but upstream of IKK (Wullaert et al., personal communication); Dok-1 and Dok-2, which are negative regulators of the Ras-Erk signaling pathway [91]; DUSP1 [92] and MKP-1 [93], which control p38 MAP kinase activation; RanGTPase, which reduces NF-κB accumulation in the nucleus [94]; and Bcl-3, which is induced by IL-10 and inhibits NF-κB binding onto its promotor sites [95] (see Figure 1).
Figure 1.
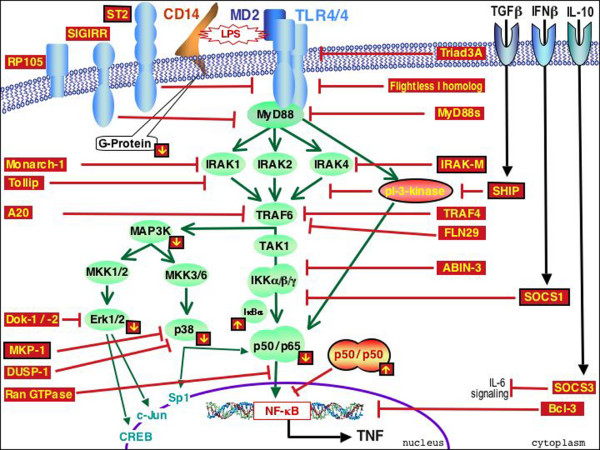
Inhibitory signals of the Toll-like receptor (TLR)4-induced MyD88-dependent signaling pathway. In addition to negative signals delivered by cytokines, cell surface receptors and numerous intracellular molecules down-regulate the TLR4-dependent signaling pathways following its activation by endotoxin (lipopolysaccharide (LPS)) (see text for further explanation). Small downwards and upwards arrows in squares indicate the down- or up-regulation, respectively, of the compound observed in endotoxin tolerant cells. Framed names of inhibitors indicate their demonstrated involvement in the endotoxin tolerance process. ABIN, A20 binding inhibitor of NF-κB activation; DUSP-1, dual specificity phosphatase 1; Erk, extracellular signal-related kinase; HO-1, heme oxygenase-1; IκB, inhibitor of κB; IRAK, IL-1 receptor associated kinase; MAPK, mitogen-activated protein kinase; MKP, MAPK phosphatase; MyD88, myeloid differentiation 88; NF-κB, nuclear factor-kappa B; pi3K, phosphatidylinositol 3-kinase; SHIP, SH1-containing inositol-5' phosphatase; SIGIRR, single immunoglobulin IL-1R-related molecule; SOCS, suppressor of cytokine signaling; STAT, signal transducer and activator of transcription; TAK1, TGFβ activating kinase 1; TGF, transforming growth factor; Tollip, Toll interacting protein; TRAF, TNF receptor associated factor.
Alteration of immune status or physiological adaptation?
Sepsis and non-infectious SIRS are associated with an exacerbated production of cytokines, as assessed by their presence in biological fluids [96]. The enhanced levels of cytokines within the bloodstream reflect the presence of activated or primed leukocytes within the different tissue compartments [97]. The local enhanced inflammatory response is a prerequisite to prevent infectious development in tissues, but failure to properly down-regulate the inflammatory process may lead to organ failure. The diminished capacity of circulating monocytes to produce cytokine upon in vitro activation displays similarities with the endotoxin tolerance phenomenon. This may represent a protective response against an overwhelming dysregulation of the pro-inflammatory process [98]. In contrast to endotoxin tolerance, which has been shown to enhance resistance to infection, the observation of an altered immune status in patients may favor an increased risk of subsequent nosocomial infections. Other defects have been reported for T cells, dendritic cells, as well as apoptosis that mainly affects immune cells. However, monocyte hyporeactivity is not a global phenomenon and some signaling pathways are unaltered and allow the cells to respond normally to certain stimuli. The terms anergy, immunodepression, or immunoparalysis are often used to qualify the immune status of sepsis patients. However, the term 'cellular reprogramming', previously proposed by Zhang and Morisson [99] to characterize endotoxin tolerance, appears to be the most appropriate one to define the events occurring among circulating leukocytes during critical illness.
Abbreviations
IFN = interferon; IL = interleukin; IRAK = IL-1 receptor associated kinase; LPS = lipopolysaccharide; MyD = myeloid differentiation; NF-κB = nuclear factor-kappa B; PBMC = peripheral blood mononuclear cell; RCA = resuscitated after cardiac arrest; SIGIRR = single immunoglobulin IL-1R-related molecule; SIRS = systemic inflammation response syndrome; SOCS = suppressor of cytokine signaling; TGF = transforming growth factor; TLR = Toll-like receptor; TNF = tumor necrosis factor; Tollip = Toll interacting protein; TRAF = TNF receptor associated factor.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
Acknowledgements
The authors are deeply grateful to their colleagues in Intensive Care Units, who, through the years, allowed their investigations, particularly Dr Christophe Adrie, Prof. Djillali Annane, Dr Jean Carlet, Prof. Jean-François Dhainaut, Dr Benoit Misset, Dr Pierre Moine, and Prof. Didier Payen. We thank Prof. Michael Pinsky for his invitation to write this review.
References
- Beeson PB. Development of tolerance to typhoid bacterial pyrogen and its abolition by reticulo-endothelial blockade. Proc Soc Exp Biol Med. 1946;61:248–250. doi: 10.3181/00379727-61-15291p. [DOI] [PubMed] [Google Scholar]
- Freeman HH. Passive transfer of tolerance to pyrogenicity of bacterial endotoxin. J Exp Med. 1960;111:453–463. doi: 10.1084/jem.111.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greisman SE, Hornick RB, Wagner HN, Jr, Woodward WE, Woodward TE. The role of endotoxin during typhoid fever and tularemia in man. J Clin Invest. 1969;48:613–629. doi: 10.1172/JCI106020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein M, Mulholland JH, Jeffery GM, Wolf SM. Malaria induced endotoxin tolerance. Proc Soc Exp Biol Med. 1965;118:283–287. doi: 10.3181/00379727-118-29820. [DOI] [PubMed] [Google Scholar]
- McCabe WR. Endotoxin tolerance. II Its occurrence in patients with pyelonephritis. J Clin Invest. 1963;42:618–625. doi: 10.1172/JCI104752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neva FA, Morgan HR. Tolerance to the action of endotoxin of enteric bacilli in patients convalescent from typhoid and paratyphoid fevers. J Lab Clin Med. 1950;35:911–922. [PubMed] [Google Scholar]
- Heyman A, Beeson PB. Influence of various disease states upon the febrile response to intravenous injection of typhoid bacterial pyrogen. J Lab Clin Med. 1949;34:1400–1403. [PubMed] [Google Scholar]
- Watson DW, Kim YB. Modification of host responses to bacterial endotoxins. I specificity of pyrogenic tolerance and the role of hypersensitivity in pyrogenicity, lethality and skin reactivity. J Exp Med. 1963;118:425–446. doi: 10.1084/jem.118.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg MA, Galanos C. Induction of tolerance to lipopolysaccharide (LPS)-D-galactosamine lethality by pre-treatment with LPS is mediated by macrophages. Infect Immun. 1988;56:1352–1357. doi: 10.1128/iai.56.5.1352-1357.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathison JC, Virca GD, Wolfson E, Tobias PS, Glaser K, Ulevitch RJ. Adaptation to bacterial lipopolysaccharide controls lipopolysaccharide-induced tumor necrosis factor production in rabbit macrophages. J Clin Invest. 1990;85:1108–1118. doi: 10.1172/JCI114542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayhane N, Fitting C, Cavaillon J-M. Dissociation of INFg from IL-12 and IL-8 during endotoxin tolerance. J Endotoxin Res. 1999;5:319–324. doi: 10.1179/096805199101531895. [DOI] [Google Scholar]
- Astiz ME, Rackow EC, Still JG, Howell ST, Cato A, Von Eschen KB, Ulrich JT, Rudbach JA, McMahon G, Vargas R, et al. Pre-treatment of normal humans with monophosphoryl lipid A induces tolerance to endotoxin: a prospective double blind, randomized, controlled trial. Crit Care Med. 1995;23:9–17. doi: 10.1097/00003246-199501000-00006. [DOI] [PubMed] [Google Scholar]
- Cavaillon JM, Pitton C, Fitting C. Endotoxin tolerance is not a LPS-specific phenomenon: partial mimicry with IL-1, IL-10 and TGFb. J Endotoxin Res. 1994;1:21–29. [Google Scholar]
- Randow F, Syrbe U, Meisel C, Krausch D, Zuckermann H, Platzer C, Volk H. Mechanism of endotoxin desensitization: involvement of interleukin-10 and transforming growth factor-b. J Exp Med. 1995;181:1887–1892. doi: 10.1084/jem.181.5.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D, Kühn R, Rajewsky K, Müller W, Menon S, Davidson N, Grünig G, Rennick D. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxin shock and the Shwartzman reaction but not endotoxin tolerance. J Clin Invest. 1995;96:2339–2347. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Fong Y, Marano MA, Gershenwald JE, Yurt RW, Moldawer LL, Lowry SF. Tolerance to endotoxin prevents mortality in infected thermal injury: association with attenuated cytokine responses. J Infect Dis. 1992;165:859–864. doi: 10.1093/infdis/165.5.859. [DOI] [PubMed] [Google Scholar]
- Colletti LM, Remick DG, Campbell DA. LPS pretreatment protects from hepatic ischemia/reperfusion. J Surg Res. 1994;57:337–343. doi: 10.1006/jsre.1994.1152. [DOI] [PubMed] [Google Scholar]
- Heemann U, Szabo A, Hamar P, Müller V, Witzke O, Lutz J, Philipp T. Lipopolysaccharide pretreatment protects from renal ischemia/reperfusion injury. Possible connection to an interleukin-6 dependent pathway. Am J Pathol. 2000;156:287–293. doi: 10.1016/S0002-9440(10)64729-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eising GP, Mao L, Schmid-Schonbein GW, Engler RL, Ross J. Effects of induced tolerance to bacterial lipopolysaccharide on myocardial infarct size in rats. Cardiovasc Res. 1996;31:73–81. doi: 10.1016/0008-6363(95)00173-5. [DOI] [PubMed] [Google Scholar]
- Ackerman M, Reuter M, Flohé S, Bahrami S, Redl H, Schade FU. Cytokine synthesis in the liver of endotoxin-tolerant and normal rats during hemorrhagic shock. J Endotoxin Res. 2001;7:105–112. doi: 10.1179/096805101101532602. [DOI] [PubMed] [Google Scholar]
- Youngner JS, Stinebring WR. Interferon appearance stimulated by endotoxin bacteria or viruses in mice pre-treated with Escherichia coli endotoxin or infected with Mycobacterium tuberculosis. Nature. 1965;208:456–458. doi: 10.1038/208456a0. [DOI] [PubMed] [Google Scholar]
- Severn A, Xu D, Doyle J, Leal LM, O'Donnell CA, Brett SJ, Moss DW, Liew FY. Pre-exposure of murine macrophages to lipopolysaccharide inhibits the induction of nitric oxide synthase and reduces leishmanicidal activity. Eur J Immunol. 1993;23:1711–1714. doi: 10.1002/eji.1830230747. [DOI] [PubMed] [Google Scholar]
- Mason CM, Dobard E, Summer WR, Nelson S. Intraportal lipopolysaccharide suppresses pulmonary antibacterial defense mechanisms. J Infec Dis. 1997;176:1293–1302. doi: 10.1086/514125. [DOI] [PubMed] [Google Scholar]
- Rayhane N, Fitting C, Lortholary O, Dromer F, Cavaillon J-M. Administration of endotoxin associated with lipopolysaccharide tolerance protects mice against fungal infection. Infect Immun. 2000;68:3748–3753. doi: 10.1128/IAI.68.6.3748-3753.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner MD, Ittner J, Bundschuh DS, N vR, Wendel A, Hartung T. Improved innate immunity of endotoxin-tolerant mice increases resistance to Salmonella enterica serovar typhimurium infection despite attenuated cytokine response. Infect Immun. 2001;69:463–471. doi: 10.1128/IAI.69.1.463-471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz C, Carlet J, Fitting C, Misset B, Bleriot JP, Cavaillon JM. Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Invest. 1991;88:1747–1754. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granowitz EV, Porat R, Mier JW, Orencole SF, Kaplanski G, Lynch EA, Ye K, Vannier E, Wolff SM, Dinarello CA. Intravenous endotoxin suppresses the cytokine response of peripheral blood mononuclear cells of healthy humans. J Immunol. 1993;151:1637–1645. [PubMed] [Google Scholar]
- Cabié A, Fitting C, Farkas J-C, Laurian C, Cormier J-M, Carlet J, Cavaillon J-M. Influence of surgery on in-vitro cytokine production by human monocytes. Cytokine. 1992;4:576–580. doi: 10.1016/1043-4666(92)90022-J. [DOI] [PubMed] [Google Scholar]
- Adib-Conquy M, Moine P, Asehnoune K, Edouard A, Espevik T, Miyake K, Werts C, Cavaillon J-M. Toll-like receptor-mediated tumor necrosis factor and interleukin-10 production differ during systemic inflammation. Am J Resp Crit Care Med. 2003;168:158–164. doi: 10.1164/rccm.200209-1077OC. [DOI] [PubMed] [Google Scholar]
- Van Deuren M, Van Der Ven-Jongekrijg H, Demacker PNM, Baterlink AKN, Van Dalen R, Sauerwein RW, Gallati H, Vannice J, van Der Meer JWM. Differential expression of proinflammatory cytokines and their inhibitors during the course of meningococcal infections. J Infect Dis. 1994;169:157–161. doi: 10.1093/infdis/169.1.157. [DOI] [PubMed] [Google Scholar]
- Marie C, Muret J, Fitting C, Payen D, Cavaillon J-M. IL-1 receptor antagonist production during infectious and noninfectious systemic inflammatory response syndrome. Crit Care Med. 2000;28:2277–2283. doi: 10.1097/00003246-200007000-00016. [DOI] [PubMed] [Google Scholar]
- Cavaillon J-M. "Septic Plasma": an immunosuppressive milieu. Am J Respir Crit Care Med. 2002;166:1417–1418. doi: 10.1164/rccm.2209003. [DOI] [PubMed] [Google Scholar]
- Cavaillon J-M, Adib-Conquy M, Cloëz-Tayarani I, Fitting C. Immunodepression in sepsis and SIRS assessed by ex vivo cytokine production is not a generalized phenomenon: a review. J Endotoxin Res. 2001;7:85–93. doi: 10.1179/096805101101532576. [DOI] [PubMed] [Google Scholar]
- Haupt W, Zirngibl H, Riese J, Stehr A, Linde HJ, Hohenberger W. Depression of tumor necrosis factor-alpha, interleukin-6, and interleukin-10 production: a reaction to the initial systemic hyperactivation in septic shock. J Invest Surg. 1997;10:349–355. doi: 10.3109/08941939709099598. [DOI] [PubMed] [Google Scholar]
- Adib-Conquy M, Adrie C, Fitting C, Gattoliat O, Beyaert R, Cavaillon JM. Up-regulation of MyD88s and SIGIRR, molecules inhibiting Toll-like receptor signaling, in monocytes from septic patients. Crit Care Med. 2006;34:2377–2385. doi: 10.1097/01.CCM.0000233875.93866.88. [DOI] [PubMed] [Google Scholar]
- Muñoz C, Misset B, Fitting C, Bleriot JP, Carlet J, Cavaillon J-M. Dissociation between plasma and monocyte-associated cytokines during sepsis. Eur J Immunol. 1991;21:2177–2184. doi: 10.1002/eji.1830210928. [DOI] [PubMed] [Google Scholar]
- Weiss M, Fischer G, Barth E, Boneberg E, Schneider EM, Georgieff M, Hartung T. Dissociation of LPS-induced monocytic ex vivo production of granulocyte colony-stimulating factor (G-CSF) and TNF-alpha in patients with septic shock. Cytokine. 2001;13:51–54. doi: 10.1006/cyto.2000.0796. [DOI] [PubMed] [Google Scholar]
- Maxime V, Fitting C, Annane D, Cavaillon J-M. Corticoids normalize leukocyte production of macrophage migration inhibitory factor in septic shock. J Infect Dis. 2005;191:138–144. doi: 10.1086/426401. [DOI] [PubMed] [Google Scholar]
- Hensler T, Hecker H, Heeg K, Heidecke CD, Bartels H, Barthlen W, Wagner H, Siewert JR, Holzmann B. Distinct mechanism of immunosuppression as a consequence of major surgery. Infect Immun. 1997;65:2283–2291. doi: 10.1128/iai.65.6.2283-2291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrie C, Adib-Conquy M, Laurent I, Monchi M, Vinsonneau C, Fitting C, Fraisse F, Dinh-Xuan AT, Carli P, Spaulding C, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a "sepsis like" syndrome. Circulation. 2002;106:562–568. doi: 10.1161/01.CIR.0000023891.80661.AD. [DOI] [PubMed] [Google Scholar]
- Rigato O, Salomao R. Impaired production of interferon-gamma and tumor necrosis factor-alpha but not of interleukin 10 in whole blood of patients with sepsis. Shock. 2003;19:113–116. doi: 10.1097/00024382-200302000-00004. [DOI] [PubMed] [Google Scholar]
- Marchant A, Alegre M, Hakim A, Piérard G, Marécaux G, Friedman G, De Groote D, Kahn R, Vincent J, Goldman M. Clinical and biological significance of interleukin-10 plasma levels in patients with septic shock. J Clin Immunol. 1995;15:265–272. doi: 10.1007/BF01540884. [DOI] [PubMed] [Google Scholar]
- Dobrovolskaia MA, Medvedev AE, Thomas KE, Cuesta N, Toshchakov V, Ren T, Cody MJ, Michalek SM, Rice NR, Vogel SN. Induction of in vitro reprogramming by Toll-like receptor (TLR)2 and TLR4 agonists in murine macrophages: effects of TLR "homotolerance" versus "heterotolerance" on NF-kappa B signaling pathway components. J Immunol. 2003;170:508–519. doi: 10.4049/jimmunol.170.1.508. [DOI] [PubMed] [Google Scholar]
- Fitting C, Dhawan S, Cavaillon JM. Compartmentalisation of endotoxin tolerance. J Infect Dis. 2004;189:1295–1303. doi: 10.1086/382657. [DOI] [PubMed] [Google Scholar]
- Mitov IG, Kropec A, Benzing A, Just H, Garotta G, Galanos C, Freudenberg M. Differential cytokine production in stimulated blood cultures from intensive care patients with bacterial infections. Infection. 1997;25:206–212. doi: 10.1007/BF01713144. [DOI] [PubMed] [Google Scholar]
- Muret J, Marie C, Fitting C, Payen D, Cavaillon J-M. Ex vivo T-lymphocyte derived cytokine production in SIRS patients is influenced by experimental procedures. Shock. 2000;13:169–174. doi: 10.1097/00024382-200003000-00001. [DOI] [PubMed] [Google Scholar]
- Wilhelm W, Grundmann U, Rensing H, Werth M, Langemeyer J, Stracke C, Dhingra D, Bauer M. Monocyte deactivation in severe human sepsis or following cardiopulmonary bypass. Shock. 2002;17:354–360. doi: 10.1097/00024382-200205000-00002. [DOI] [PubMed] [Google Scholar]
- Constantian M. Association of sepsis with an immunosuppressive polypeptide in the serum of burn patients. Ann Surg. 1978;188:209–215. doi: 10.1097/00000658-197808000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins JM, Kuijper EJ, Mevissen ML, Speelman P, van Deventer SJ. Release of tumor necrosis factor alpha and interleukin 6 during antibiotic killing of Escherichia coli in whole blood: influence of antibiotic class, antibiotic concentration, and presence of septic serum. Infect Immun. 1995;63:2236–2242. doi: 10.1128/iai.63.6.2236-2242.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinas G, Bloesch D, Kaufmann M, Keller U, Dayer JM. Induction of plasma inhibitors of interleukin 1 and TNF-alpha activity by endotoxin administration to normal humans. Am J Physiol. 1990;259:R993–R997. doi: 10.1152/ajpregu.1990.259.5.R993. [DOI] [PubMed] [Google Scholar]
- Ronco C, Brendolan A, Lonnemann G, Bellomo R, Piccinni P, Digito A, Dan M, Irone M, La Greca G, Inguaggiato P, et al. A pilot study of coupled plasma filtration with adsorption in septic shock. Crit Care Med. 2002;30:1250–1255. doi: 10.1097/00003246-200206000-00015. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P, Osnes L, Øvstebø R, Joø GB, Westwik AB, Kierulf P. Net inflammatory capacity of human septic shock plasma evaluated by a monocyte-based target cell assay: identification of interleukin-10 as a major functional deactivator of human monocytes. J Exp Med. 1996;184:51–60. doi: 10.1084/jem.184.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie C, Fitting C, Muret J, Payen D, Cavaillon J-M. Interleukin-8 production in whole blood assays: is interleukin-10 responsible for the downregulation observed in sepsis? Cytokine. 2000;12:55–61. doi: 10.1006/cyto.1999.0517. [DOI] [PubMed] [Google Scholar]
- Ayala A, Knotts JB, Ertel W, Perrin MM, Morrison MH, Chaudry IH. Role of interleukin 6 and transforming growth factor-beta in the induction of depressed splenocyte responses following sepsis. Arch Surg. 1993;128:89–94. doi: 10.1001/archsurg.1993.01420130101015. [DOI] [PubMed] [Google Scholar]
- Miller-Graziano CL, Szabo G, Griffey K, Mehta B, Kodys K, Catalano D. Role of elevated monocyte transforming growth factor β production in post-trauma immunosuppression. J Clin Immunol. 1991;11:95–102. doi: 10.1007/BF00917745. [DOI] [PubMed] [Google Scholar]
- Chen W, Frank M, Jin W, Wahl S. TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity. 2001;14:715–725. doi: 10.1016/S1074-7613(01)00147-9. [DOI] [PubMed] [Google Scholar]
- Jones SB, Westfall MV, Sayeed MM. Plasma catecholamines during E. coli bacteremia in conscious rats. Am J Physiol. 1988;254:R470–477. doi: 10.1152/ajpregu.1988.254.3.R470. [DOI] [PubMed] [Google Scholar]
- Luger TA, Kalden DH, Scholzen TE, Brzoska T. A melanocyte stimulating hormone as a mediator of tolerance induction. Pathobiology. 1999;67:318–321. doi: 10.1159/000028089. [DOI] [PubMed] [Google Scholar]
- Delgado M, Pozo D, Martinez C, Leceta J, Calvo JR, Ganea D, Gomariz RP. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit endotoxin-induced TNFa production by macrophages: in vitro and in vivo studies. J Immunol. 1999;162:2358–2367. [PubMed] [Google Scholar]
- Annane D, Cavaillon JM. Corticosteroids in sepsis: from bench to bedside? Shock. 2003;20:197–207. doi: 10.1097/01.shk.0000079423.72656.2f. [DOI] [PubMed] [Google Scholar]
- Choudhry MA, Ahmad S, Ahmed Z, Sayeed MM. Prostaglandin E2 down-regulation of T cell IL-2 production is independent of IL-10 during Gram negative sepsis. Immunol Lett. 1999;67:125–130. doi: 10.1016/S0165-2478(99)00003-6. [DOI] [PubMed] [Google Scholar]
- Njemini R, Lambert M, Demanet C, Mets T. Elevated serum heat-shock protein 70 levels in patients with acute infection: use of an optimized enzyme-linked immunosorbent assay. Scand J Immunol. 2003;58:664–669. doi: 10.1111/j.1365-3083.2003.01341.x. [DOI] [PubMed] [Google Scholar]
- Ding X, Fernandez-Prada C, Bhattacharjee A, Hoover D. Over-expression of hsp-70 inhibits bacterial lipopolysaccharide-induced production of cytokines in human monocyte-derived macrophages. Cytokine. 2001;16:210–219. doi: 10.1006/cyto.2001.0959. [DOI] [PubMed] [Google Scholar]
- Cavaillon JM, Adrie C, Fitting C, Adib-Conquy M. Endotoxin tolerance: is there a clinical relevance? J Endotoxin Res. 2003;9:101–107. doi: 10.1179/096805103125001487. [DOI] [PubMed] [Google Scholar]
- Kitchens RL, Thompson PA, Viriyakosol S, O'Keefe GE, Munford RS. Plasma CD14 decreases monocyte responses to LPS by transferring cell-bound LPS to plasma lipoproteins. J Clin Invest. 2001;108:485–493. doi: 10.1172/JCI200113139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugdenhil ACE, Snoeck AMP, van't Veer C, Greve JWM, Buurman WA. LPS-binding protein circulates in association with apoB-containing lipoproteins and enhances endotoxin-LDL/VLDL interaction. J Clin Invest. 2001;107:225–234. doi: 10.1172/JCI10832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillon JM, Fitting C, Haeffner-Cavaillon N, Kirsch SJ, Warren HS. Cytokine response by monocytes and macrophages to free and lipoprotein-bound lipopolysaccharide. Infect Immun. 1990;58:2375–2382. doi: 10.1128/iai.58.7.2375-2382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren HS, Knights CV, Siber GR. Neutralization and lipoprotein binding of lipopolysaccharides in tolerant rabbit serum. J Infect Dis. 1986;154:784–791. doi: 10.1093/infdis/154.5.784. [DOI] [PubMed] [Google Scholar]
- Majetschak M, Krehmeier U, Bardenheuer M, Denz C, Quintel M, Voggenreiter G, Obertacke U. Extracellular ubiquitin inhibits the TNF-alpha response to endotoxin in peripheral blood mononuclear cells and regulates endotoxin hyporesponsiveness in critical illness. Blood. 2003;101:1882–1890. doi: 10.1182/blood-2002-03-0918. [DOI] [PubMed] [Google Scholar]
- Blackwell TS, Blackwell TR, Christman JW. Induction of endotoxin tolerance depletes nuclear factor-kB and suppresses its activation in rat alveolar macrophages. J Leuk Biol. 1997;62:885–891. doi: 10.1002/jlb.62.6.885. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock HWL, Wedel A, Schraut W, Ströbel M, Wendelgass P, Sterndorf T, Bäuerle PA, Haas JG, Riethmüller G. Tolerance to lipopolysaccharide involves mobilization of nuclear factor κB with predominance of p50 homodimers. J Biol Chem. 1994;269:17001–17004. [PubMed] [Google Scholar]
- Adib-Conquy M, Adrie C, Moine P, Asehnoune K, Fitting C, Pinsky MR, Dhainaut J-F, Cavaillon J-M. NF-κB expression in mononuclear cells of septic patients resembles that observed in LPS-tolerance. Am J Respir Crit Care Med. 2000;162:1877–1883. doi: 10.1164/ajrccm.162.5.2003058. [DOI] [PubMed] [Google Scholar]
- Adib-Conquy M, Asehnoune K, Moine P, Cavaillon J-M. Long term impaired expression of nuclear factor-kB and IkBa in peripheral blood mononuclear cells of patients with major trauma. J Leuk Biol. 2001;70:30–38. [PubMed] [Google Scholar]
- Kobayashi K, Hernandez LD, Galan JE, Janeway CAJ, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/S0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- Escoll P, del Fresno C, Garcia L, Valles G, Lendinez M, Arnalich F, Lopez-Collazo E. Rapid up-regulation of IRAK-M expression following a second endotoxin challenge in human monocytes and in monocytes isolated from septic patients. Biochem Biophys Res Commun. 2003;311:465–472. doi: 10.1016/j.bbrc.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24:358–363. doi: 10.1016/S1471-4906(03)00139-X. [DOI] [PubMed] [Google Scholar]
- Learn CA, Boger MS, Li L, McCall CE. The phosphatidylinositol 3 kinase pathway selectively controls sIL-1ra not interleukin-1β production in the septic leukocytes. J Biol Chem. 2001;276:20234–20239. doi: 10.1074/jbc.M100316200. [DOI] [PubMed] [Google Scholar]
- Zhang G, Ghosh S. Negative regulation of toll-like receptor-mediated signaling by Tollip. J Biol Chem. 2002;277:7059–7065. doi: 10.1074/jbc.M109537200. [DOI] [PubMed] [Google Scholar]
- Janssens S, Burns K, Tschopp J, Beyaert R. Regulation of interleukin-1- and lipopolysaccharide-induced NF-kappaB activation by alternative splicing of MyD88. Curr Biol. 2002;12:467–471. doi: 10.1016/S0960-9822(02)00712-1. [DOI] [PubMed] [Google Scholar]
- Wald D, Qin J, Zhao Z, Qian Y, Naramura M, Tian L, Towne J, Sims JE, Stark GR, Li X. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2003;4:920–927. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- Nakagawa R, Naka T, Tsutsui H, Fujimoto M, Kimura A, Abe T, Seki E, Sato S, Takeuchi O, Takeda K, et al. SOCS-1 participates in negative regulation of LPS responses. Immunity. 2002;17:677–687. doi: 10.1016/S1074-7613(02)00449-1. [DOI] [PubMed] [Google Scholar]
- Divanovic S, Trompette A, Atabani SF, Madan R, Golenbock DT, Visintin A, Finberg RW, Tarakhovsky A, Vogel SN, Belkaid Y, et al. Inhibition of TLR-4/MD-2 signaling by RP105/MD-1. J Endotoxin Res. 2005;11:363–368. doi: 10.1179/096805105X67300. [DOI] [PubMed] [Google Scholar]
- Brint EK, Xu D, Liu H, Dunne A, McKenzie ANJ, O'Neill LAJ, Liew FY. ST2 is an inhibitor of interleukin 1 receptor and toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat Immunol. 2004;5:373–379. doi: 10.1038/ni1050. [DOI] [PubMed] [Google Scholar]
- Chuang TH, Ulevitch RJ. Triad3A, an E3 ubiquitin-protein ligase regulating Toll-like receptors. Nat Immunol. 2004;5:495–502. doi: 10.1038/ni1066. [DOI] [PubMed] [Google Scholar]
- Wang T, Chuang TH, Ronni T, Gu S, Du YC, Cai H, Sun HQ, Yin HL, Chen X. Flightless I homolog negatively modulates the TLR pathway. J Immunol. 2006;176:1355–1362. doi: 10.4049/jimmunol.176.3.1355. [DOI] [PubMed] [Google Scholar]
- Williams KL, Lich JD, Duncan JA, Reed W, Rallabhandi P, Moore C, Kurtz S, Coffield VM, Accavitti-Loper MA, Su L, et al. The CATERPILLER protein monarch-1 is an antagonist of toll-like receptor-, tumor necrosis factor alpha-, and Mycobacterium tuberculosis-induced pro-inflammatory signals. J Biol Chem. 2005;280:39914–39924. doi: 10.1074/jbc.M502820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5:1052–1060. doi: 10.1038/ni1110. [DOI] [PubMed] [Google Scholar]
- Rauh MJ, Sly LM, Kalesnikoff J, Hughes MR, Cao LP, Lam V, Krystal G. The role of SHIP1 in macrophage programming and activation. Biochem Soc Trans. 2004;32:785–788. doi: 10.1042/BST0320785. [DOI] [PubMed] [Google Scholar]
- Takeshita F, Ishii KJ, Kobiyama K, Kojima Y, Coban C, Sasaki S, Ishii N, Klinman DM, Okuda K, Akira S, et al. TRAF4 acts as a silencer in TLR-mediated signaling through the association with TRAF6 and TRIF. Eur J Immunol. 2005;35:2477–2485. doi: 10.1002/eji.200526151. [DOI] [PubMed] [Google Scholar]
- Mashima R, Saeki K, Aki D, Minoda Y, Takaki H, Sanada T, Kobayashi T, Aburatani H, Yamanashi Y, Yoshimura A. FLN29, a novel interferon- and LPS-inducible gene acting as a negative regulator of toll-like receptor signaling. J Biol Chem. 2005;280:41289–41297. doi: 10.1074/jbc.M508221200. [DOI] [PubMed] [Google Scholar]
- Shinohara H, Inoue A, Toyama-Sorimachi N, Nagai Y, Yasuda T, Suzuki H, Horai R, Iwakura Y, Yamamoto T, Karasuyama H, et al. Dok-1 and Dok-2 are negative regulators of lipopolysaccharide-induced signaling. J Exp Med. 2005;201:333–339. doi: 10.1084/jem.20041817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer M, Mages J, Dietrich H, Servatius A, Howells N, Cato AC, Lang R. Dual specificity phosphatase 1 (DUSP1) regulates a subset of LPS-induced genes and protects mice from lethal endotoxin shock. J Exp Med. 2006;203:15–20. doi: 10.1084/jem.20051753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Wang X, Nelin LD, Yao Y, Matta R, Manson ME, Baliga RS, Meng X, Smith CV, Bauer JA, et al. MAP kinase phosphatase 1 controls innate immune responses and suppresses endotoxic shock. J Exp Med. 2006;203:131–140. doi: 10.1084/jem.20051794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Yuan Q, Sultzer BM, Chung SW, Wong PM. The involvement of Ran GTPase in lipopolysaccharide endotoxin-induced responses. J Endotoxin Res. 2001;7:53–56. doi: 10.1179/096805101101532549. [DOI] [PubMed] [Google Scholar]
- Kuwert EK, Marcus I, Werner J, Iwand A, Thraenhart O. Some experiences with human diploid cell strain-(HDCS) rabies vaccine in pre- and post-exposure vaccinated humans. Dev Biol Stand. 1978;40:79–88. [PubMed] [Google Scholar]
- Cavaillon JM, Adib-Conquy M, Fitting C, Adrie C, Payen D. Cytokine cascade in sepsis. Scand J Infect Dis. 2003;35:535–544. doi: 10.1080/00365540310015935. [DOI] [PubMed] [Google Scholar]
- Cavaillon J, Annane D. Compartmentalization of the inflammatory response in sepsis and SIRS. J Endotoxin Res. 2006 doi: 10.1179/096805106X102246. [DOI] [PubMed] [Google Scholar]
- Munford RS, Pugin J. Normal response to injury prevent systemic inflammation and can be immunosuppressive. Am J Respir Crit Care Med. 2001;163:316–321. doi: 10.1164/ajrccm.163.2.2007102. [DOI] [PubMed] [Google Scholar]
- Zhang X, Morrison DC. Lipopolysaccharide structure-function relationship in activation versus reprogramming of mouse peritoneal macrophages. J Leukoc Biol. 1993;54:444–450. doi: 10.1002/jlb.54.5.444. [DOI] [PubMed] [Google Scholar]


