Abstract
Background and Aims: Increased generation of reactive oxygen species and mitochondrial dysfunction may underlie the pathophysiology of Friedreich's ataxia, the most common inherited ataxia, due to GAA expansion in a gene coding for a mitochondrial protein (frataxin), implicated in the regulation of iron metabolism. Because iron overload would cause oxidative stress in Friedreich's ataxia, we investigated the enzyme antioxidant system in the blood of 14 patients by determining superoxide dismutase, glutathione peroxidase, and glutathione trasferase catalytic activities. We also studied the glutathione S-transferase genotype polymorphism in order to evaluate its possible influence on enzyme activity.
Methods: Blood samples were obtained from 14 unrelated patients with Friedreich's ataxia and 21 age matched healthy subjects. Antioxidant enzyme determinations were spectrophotometrically assayed using specific substrates; the glutathione S-transferase genotype polymorphism was analysed by endonuclease restriction mapping of exon 5 and 6 amplification products.
Results: There was a significant elevation of the superoxide dismutase/glutathione peroxidase activity ratio (0.037 (0.01) v 0.025 (0.008) of controls) and an 83% rise of glutathione transferase specific activity (0.22 (0.1) v 0.12 (0.03) nmol/min/mg protein) in blood of patients with Friedreich's ataxia than in the controls. The genotype polymorphism of glutathione S-transferase enzyme did not show any relevant differences when compared to that of healthy subjects.
Conclusions: Data show an impairment in vivo of antioxidant enzymes in patients with Friedreich's ataxia and provide evidence of an increased sensitivity to oxidative stress, supporting a consistent role of free radical cytotoxicity in the pathophysiology of the disease.
Full Text
The Full Text of this article is available as a PDF (146.9 KB).
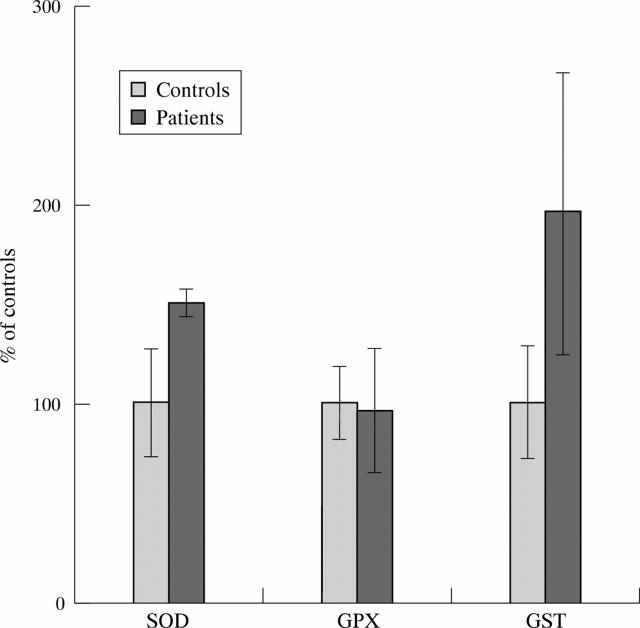
Figure 1 .
SOD, GPX, and GSTP1-1 enzyme activities in erythrocytes of 14 FRDA patients. Values are expressed as % of age matched controls (n = 21).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler V., Yin Z., Fuchs S. Y., Benezra M., Rosario L., Tew K. D., Pincus M. R., Sardana M., Henderson C. J., Wolf C. R. Regulation of JNK signaling by GSTp. EMBO J. 1999 Mar 1;18(5):1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali-Osman F., Akande O., Antoun G., Mao J. X., Buolamwini J. Molecular cloning, characterization, and expression in Escherichia coli of full-length cDNAs of three human glutathione S-transferase Pi gene variants. Evidence for differential catalytic activity of the encoded proteins. J Biol Chem. 1997 Apr 11;272(15):10004–10012. doi: 10.1074/jbc.272.15.10004. [DOI] [PubMed] [Google Scholar]
- Amstad P., Moret R., Cerutti P. Glutathione peroxidase compensates for the hypersensitivity of Cu,Zn-superoxide dismutase overproducers to oxidant stress. J Biol Chem. 1994 Jan 21;269(3):1606–1609. [PubMed] [Google Scholar]
- Bar-Peled O., Korkotian E., Segal M., Groner Y. Constitutive overexpression of Cu/Zn superoxide dismutase exacerbates kainic acid-induced apoptosis of transgenic-Cu/Zn superoxide dismutase neurons. Proc Natl Acad Sci U S A. 1996 Aug 6;93(16):8530–8535. doi: 10.1073/pnas.93.16.8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley J. L., Blake J. C., Chamberlain S., Thomas P. K., Cooper J. M., Schapira A. H. Clinical, biochemical and molecular genetic correlations in Friedreich's ataxia. Hum Mol Genet. 2000 Jan 22;9(2):275–282. doi: 10.1093/hmg/9.2.275. [DOI] [PubMed] [Google Scholar]
- Delatycki M. B., Camakaris J., Brooks H., Evans-Whipp T., Thorburn D. R., Williamson R., Forrest S. M. Direct evidence that mitochondrial iron accumulation occurs in Friedreich ataxia. Ann Neurol. 1999 May;45(5):673–675. [PubMed] [Google Scholar]
- Emond M., Lepage G., Vanasse M., Pandolfo M. Increased levels of plasma malondialdehyde in Friedreich ataxia. Neurology. 2000 Dec 12;55(11):1752–1753. doi: 10.1212/wnl.55.11.1752. [DOI] [PubMed] [Google Scholar]
- Franco A. A., Odom R. S., Rando T. A. Regulation of antioxidant enzyme gene expression in response to oxidative stress and during differentiation of mouse skeletal muscle. Free Radic Biol Med. 1999 Nov;27(9-10):1122–1132. doi: 10.1016/s0891-5849(99)00166-5. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide anion radical (O2-.), superoxide dismutases, and related matters. J Biol Chem. 1997 Jul 25;272(30):18515–18517. doi: 10.1074/jbc.272.30.18515. [DOI] [PubMed] [Google Scholar]
- Gorman A. M., McGowan A., O'Neill C., Cotter T. Oxidative stress and apoptosis in neurodegeneration. J Neurol Sci. 1996 Aug;139 (Suppl):45–52. doi: 10.1016/0022-510x(96)00097-4. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Jakoby W. B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Harding A. E. Classification of the hereditary ataxias and paraplegias. Lancet. 1983 May 21;1(8334):1151–1155. doi: 10.1016/s0140-6736(83)92879-9. [DOI] [PubMed] [Google Scholar]
- Hayes J. D., McLellan L. I. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic Res. 1999 Oct;31(4):273–300. doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- Hayes J. D., Strange R. C. Glutathione S-transferase polymorphisms and their biological consequences. Pharmacology. 2000 Sep;61(3):154–166. doi: 10.1159/000028396. [DOI] [PubMed] [Google Scholar]
- Knight S. A., Kim R., Pain D., Dancis A. The yeast connection to Friedreich ataxia. Am J Hum Genet. 1999 Feb;64(2):365–371. doi: 10.1086/302270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodi R., Hart P. E., Rajagopalan B., Taylor D. J., Crilley J. G., Bradley J. L., Blamire A. M., Manners D., Styles P., Schapira A. H. Antioxidant treatment improves in vivo cardiac and skeletal muscle bioenergetics in patients with Friedreich's ataxia. Ann Neurol. 2001 May;49(5):590–596. [PubMed] [Google Scholar]
- Mehta J. L., Li D. Epinephrine upregulates superoxide dismutase in human coronary artery endothelial cells. Free Radic Biol Med. 2001 Jan 15;30(2):148–153. doi: 10.1016/s0891-5849(00)00442-1. [DOI] [PubMed] [Google Scholar]
- Michiels C., Raes M., Toussaint O., Remacle J. Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free Radic Biol Med. 1994 Sep;17(3):235–248. doi: 10.1016/0891-5849(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Pandolfo M. Molecular genetics and pathogenesis of Friedreich ataxia. Neuromuscul Disord. 1998 Aug;8(6):409–415. doi: 10.1016/s0960-8966(98)00039-x. [DOI] [PubMed] [Google Scholar]
- Pastor M. C., Sierra C., Doladé M., Navarro E., Brandi N., Cabré E., Mira A., Serés A. Antioxidant enzymes and fatty acid status in erythrocytes of Down's syndrome patients. Clin Chem. 1998 May;44(5):924–929. [PubMed] [Google Scholar]
- Puccio H., Koenig M. Recent advances in the molecular pathogenesis of Friedreich ataxia. Hum Mol Genet. 2000 Apr 12;9(6):887–892. doi: 10.1093/hmg/9.6.887. [DOI] [PubMed] [Google Scholar]
- Radisky D. C., Babcock M. C., Kaplan J. The yeast frataxin homologue mediates mitochondrial iron efflux. Evidence for a mitochondrial iron cycle. J Biol Chem. 1999 Feb 19;274(8):4497–4499. doi: 10.1074/jbc.274.8.4497. [DOI] [PubMed] [Google Scholar]
- Rötig A., de Lonlay P., Chretien D., Foury F., Koenig M., Sidi D., Munnich A., Rustin P. Aconitase and mitochondrial iron-sulphur protein deficiency in Friedreich ataxia. Nat Genet. 1997 Oct;17(2):215–217. doi: 10.1038/ng1097-215. [DOI] [PubMed] [Google Scholar]
- Schapira A. H. Mitochondrial involvement in Parkinson's disease, Huntington's disease, hereditary spastic paraplegia and Friedreich's ataxia. Biochim Biophys Acta. 1999 Feb 9;1410(2):159–170. doi: 10.1016/s0005-2728(98)00164-9. [DOI] [PubMed] [Google Scholar]
- Schulz J. B., Dehmer T., Schöls L., Mende H., Hardt C., Vorgerd M., Bürk K., Matson W., Dichgans J., Beal M. F. Oxidative stress in patients with Friedreich ataxia. Neurology. 2000 Dec 12;55(11):1719–1721. doi: 10.1212/wnl.55.11.1719. [DOI] [PubMed] [Google Scholar]
- Sun A. Y., Chen Y. M. Oxidative stress and neurodegenerative disorders. J Biomed Sci. 1998 Nov-Dec;5(6):401–414. doi: 10.1007/BF02255928. [DOI] [PubMed] [Google Scholar]
- Sundberg K., Johansson A. S., Stenberg G., Widersten M., Seidel A., Mannervik B., Jernström B. Differences in the catalytic efficiencies of allelic variants of glutathione transferase P1-1 towards carcinogenic diol epoxides of polycyclic aromatic hydrocarbons. Carcinogenesis. 1998 Mar;19(3):433–436. doi: 10.1093/carcin/19.3.433. [DOI] [PubMed] [Google Scholar]
- Tabrizi S. J., Workman J., Hart P. E., Mangiarini L., Mahal A., Bates G., Cooper J. M., Schapira A. H. Mitochondrial dysfunction and free radical damage in the Huntington R6/2 transgenic mouse. Ann Neurol. 2000 Jan;47(1):80–86. doi: 10.1002/1531-8249(200001)47:1<80::aid-ana13>3.3.co;2-b. [DOI] [PubMed] [Google Scholar]
- Warner H. R. Superoxide dismutase, aging, and degenerative disease. Free Radic Biol Med. 1994 Sep;17(3):249–258. doi: 10.1016/0891-5849(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Wong A., Yang J., Cavadini P., Gellera C., Lonnerdal B., Taroni F., Cortopassi G. The Friedreich's ataxia mutation confers cellular sensitivity to oxidant stress which is rescued by chelators of iron and calcium and inhibitors of apoptosis. Hum Mol Genet. 1999 Mar;8(3):425–430. doi: 10.1093/hmg/8.3.425. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Elwell J. H., Oberley L. W., Goeddel D. V. Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell. 1989 Sep 8;58(5):923–931. doi: 10.1016/0092-8674(89)90944-6. [DOI] [PubMed] [Google Scholar]
- Xia C., Hu J., Ketterer B., Taylor J. B. The organization of the human GSTP1-1 gene promoter and its response to retinoic acid and cellular redox status. Biochem J. 1996 Jan 1;313(Pt 1):155–161. doi: 10.1042/bj3130155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambon M. C., Stockton J. D., Clewley J. P., Fleming D. M. Contribution of influenza and respiratory syncytial virus to community cases of influenza-like illness: an observational study. Lancet. 2001 Oct 27;358(9291):1410–1416. doi: 10.1016/s0140-6736(01)06528-x. [DOI] [PubMed] [Google Scholar]
- Zhang K., Mack P., Wong K. P. Glutathione-related mechanisms in cellular resistance to anticancer drugs. Int J Oncol. 1998 Apr;12(4):871–882. doi: 10.3892/ijo.12.4.871. [DOI] [PubMed] [Google Scholar]
- Zimniak P., Nanduri B., Pikuła S., Bandorowicz-Pikuła J., Singhal S. S., Srivastava S. K., Awasthi S., Awasthi Y. C. Naturally occurring human glutathione S-transferase GSTP1-1 isoforms with isoleucine and valine in position 104 differ in enzymic properties. Eur J Biochem. 1994 Sep 15;224(3):893–899. doi: 10.1111/j.1432-1033.1994.00893.x. [DOI] [PubMed] [Google Scholar]
- de Haan J. B., Cristiano F., Iannello R., Bladier C., Kelner M. J., Kola I. Elevation in the ratio of Cu/Zn-superoxide dismutase to glutathione peroxidase activity induces features of cellular senescence and this effect is mediated by hydrogen peroxide. Hum Mol Genet. 1996 Feb;5(2):283–292. doi: 10.1093/hmg/5.2.283. [DOI] [PubMed] [Google Scholar]