Abstract
The vagus nerve is an important source of afferent information about visceral states and it provides input to the locus coeruleus (LC), the major source of norepinephrine (NE) in the brain. It has been suggested that the effects of electrical stimulation of the vagus nerve on learning and memory, mood, seizure suppression, and recovery of function following brain damage are mediated, in part, by the release of brain NE. The hypothesis that left vagus nerve stimulation (VNS) at the cervical level results in increased extracellular NE concentrations in the cortex and hippocampus was tested at four stimulus intensities 0.0, 0.25, 0.5, and 1.0 mA. Stimulation at 0.0 and 0.25 mA had no effect on NE concentrations, while the 0.5 mA stimulation increased NE concentrations significantly in the hippocampus (23%), but not the cortex. However, 1.0 mA stimulation significantly increased NE concentrations in both the cortex (39%) and hippocampus (28%) bilaterally. The increases in NE were transient and confined to the stimulation periods. VNS did not alter NE concentrations in either structure during the inter-stimulation baseline periods. No differences were observed between NE levels in the initial baseline and the post-stimulation baselines. These findings support the hypothesis that VNS increases extracellular NE concentrations in both the hippocampus and cortex.
Keywords: (6) norepinephrine, vagus nerve stimulation, epilepsy, locus coeruleus, depression, memory
Introduction
Converging anatomical, electrophysiological, biochemical, and behavioral, evidence increasingly supports the hypothesis that visceral afferents of the vagus nerve exert a functionally significant influence on the CNS through the modulation of monoaminergic systems. Moreover, the capacity of vagus nerve stimulation (VNS) to suppress seizures, improve functional recovery following experimental traumatic brain injury, ameliorate depression, and enhance learning and memory, is thought to result, in part, from the release of norepinephrine (NE) in the terminal fields of the locus coeruleus (LC). Hence, characterizing the effect of VNS on extracellular NE concentrations in the hippocampus and cortex is of interest both experimentally and clinically.
Vagus nerve – locus coeruleus connection
Vagal afferents project to the nucleus of the solitary tract (NTS) which, in turn, has widespread projections to brainstem and forebrain structures. The NTS also projects both directly and indirectly to the LC (Van Bockstaele et al., 1999). Indeed, several lines of evidence support the hypothesis that VNS activates neurons in the LC. For example, electrical stimulation of the vagus nerve in rats has been shown to increase the firing rate and c-fos expression of LC neurons in rats (Groves et al., 2005; Takigawa and Mogenson, 1977; Naritoku et al., 1995). Neurons of the LC project to the hippocampus via the dorsal bundle and are the sole source of hippocampal NE (Loy et al., 1980). The LC also provides the majority of noradrenergic innervation to the cortex. LC projections to cells of the cerebral cortex are predominantly ipsilateral; however, LC projections to the cerebellum are bilateral (Loughlin et al., 1982, 1986).
Despite the fact that VNS is typically administered unilaterally, c-fos (Naritoku et al., 1995), EEG, and neuroimaging (Henry et al., 1998; 2004) studies all reveal changes in CNS activity that are both ipsilateral and bilateral depending on the particular brain region being studied. Although VNS typically involves stimulation of the left vagus nerve at the cervical level, Zabara (1992) reported right and left VNS to be equally effective in controlling seizures in dogs; and more recently, stimulation of the right vagus nerve has been shown to be as effective in suppressing seizures in rats as stimulation of the left vagus (Krahl et al., 2003). It is not clear whether activation of the LC via vagal afferents would be expected to produce uniform release of NE throughout its terminal fields. However, if NE is indeed responsible for the antiepileptic effect of VNS, then NE release would be expected to be bilateral following unilateral stimulation.
VNS in epilepsy
The anticonvulsant effect of VNS on chronic refractory epilepsy was first reported in 1993 (Michael et al., 1993). Since that time numerous clinical trials have confirmed the effectiveness of VNS in controlling seizures (Ben-Menachem 1996; 2002; Handforth et al., 1998; Morris and Mueller, 1999; Boon, et al., 2002; Wheless and Baumgartner, 2004). Moreover, VNS exerts anticonvulsant effects in a variety of experimental animal models of epilepsy including rat (Takaya et al., 1996), dog (Zabara, 1992), and cat models (Fernandez-Guardiola et al., 1999). In addition, VNS suppresses seizures in a variety of populations including children (Crumrine, 2000; Amar et al., 2001; Wakai and Kotagal, 2001; Nagarajan et al., 2002; Kossoff and Pyzik, 2004), adolescents (Crumrine, 2000; Wakai and Kotagal, 2001; Nagarajan et al., 2002), adults (Tanganelli et al., 2002; Chavel et al., 2003; Koszewski et al., 2003; Holmes et al., 2004; Hui et al., 2004), the aged (Sirven et al., 2000) and, recently, in low IQ residents of long-term care facilities (Huf et al., 2005). The hypothesis that VNS exerts its effects through the release of NE is supported by studies showing that lesions of the LC block the anticonvulsant effects of VNS in rats (Krahl et al., 1998).
VNS in Depression
Dysfunction of the noradrenergic system has long been implicated in depression (see Leonard, 1997 for review). VNS was shown to exert antidepressant effects in a rat model of depression with an efficacy similar to desipramine or electroconvulsive shock (Krahl et al., 2004; George et al., 2005). Initial clinical studies suggest depression in humans may be responsive to VNS as well (Bolwig, 2003; Carpenter et al., 2003; Armitage et al, 2003; Hoppe et al., 2001; Harden, et al., 2000; Macritchie and Young, 2001; Topfer and Hailey, 2001; Marangell et al. 2002; George et al., 2000; Elger, et al., 2000; Rush, et al., 2000). Moreover, the vagus nerve stimulator has recently been approved for the treatment of intractable depression by the FDA.
VNS in CNS injury
The noradrenergic system has also been shown to play a role in recovery from injury in a variety of experimental models of traumatic brain injury including ablation (Feeney and Hovda, 1983; Hovda, et al., 1987; Kikuchi et al., 1999), contusion (Dunn-Meynell et al., 1994; Krobert et al., 1994; Levin et al., 1995; Queen et al., 1997), and fluid percussion (Boyeson, and Feeney, 1990; Prasad et al., 1992; McIntosh et al., 1994). In general, treatments that enhance the synaptic effects of NE improve the rate or amount of recovery following brain injury (Meyer et al, 1963; Feeney et al., 1981; Feeney et al., 1982; Feeney and Hovda, 1983; Hovda et al., 1987; 1989; Boyeson et al., 1992; Feeney et al., 1993; Queen et al., 1997; Kikuchi et al., 1999; Kikuchi et al., 2000). Furthermore, amphetamine and NE reuptake inhibitors have been demonstrated to improve function in humans following stroke (Boyeson, 1996). Conversely, treatment with a NE antagonist impairs recovery and may even reinstate deficits in rats following CNS injury (Goldstein and Davis, 1990). VNS (0.5mA, 30 s duration, every 30 min.) that begins 2 hours following fluid percussion injury reduces the degree of initial impairment and enhances both the rate and final level of motor and cognitive recovery in rats (Smith et al., 2005). VNS also reduces the formation of edema when measured 24 hours post-injury (unpublished data).
VNS in memory and learning
Peripheral state, especially arousal, modulates learning and memory in a variety of tasks including inhibitory avoidance (Clark et al., 1998), spatial navigation (Williams and McGaugh, 1993), and verbal memory in humans (Nielson and Jensen, 1995). These effects can be attenuated by vagotomy, drugs that diminish the effects of arousal, or lesions of the NTS (Williams and McGaugh, 1993). Further, electrical stimulation of the vagus results in improved performance on memory tasks in rats (Clark et al., 1995; Clark et al., 1998) and in humans (Clark et al, 1999). More recently in our laboratory, VNS has been shown to facilitate the induction of early and late LTP in the dentate gyrus of freely moving rats, presumably through the capacity of VNS to increase noradrenergic neurotransmission in the CNS.
Hence, anatomical, biochemical, electrophysiological, and behavioral findings converge to strongly suggest that VNS should increase the release of NE in the terminal fields of the LC, and that many of the effects of VNS are attributable to the release of NE. While VNS has been reported to increase NE in the amygdala (Hassert et al., 2004), which receives input from both the lateral tegmental system and LC (Moore, 1984), direct evidence of increased NE concentrations structures innervated solely or predominantly by the LC has not been previously reported. In the present study, we employed in vivo microdialysis to determine whether VNS enhances the release of NE in the cerebral cortex and hippocampus of freely moving rats
Results
Vagus nerve stimulation (VNS) caused no observable behavioral response to the 0.25 mA stimulus intensity, but produced a brief change in the respiratory pattern (i.e. rhythm change) during the first few stimulation deliveries at the 0.5 and 1.0 mA levels. Freezing behavior was also occasionally observed during the first few stimulus deliveries as well as an occasional muscle twitch in the area of the neck adjacent to the electrode.
In hippocampus the 0.0, 0.25, 0.5, and 1.0 mA levels of VNS produced percent changes in extracellular NE of −6.75, 9.43, 23.08, and 28.45, respectively, when compared to baseline (Fig. 1). These percent changes in NE concentrations varied significantly within subjects on the basis of level of stimulation [F(3,69) =4.774, p<0.008]. Pairwise comparisons yielded significant differences between sham stimulation (0.0 mA) and 0.5 (p<0.05) and 1.0 mA (p<0.001) levels of stimulation.
Figure 1.
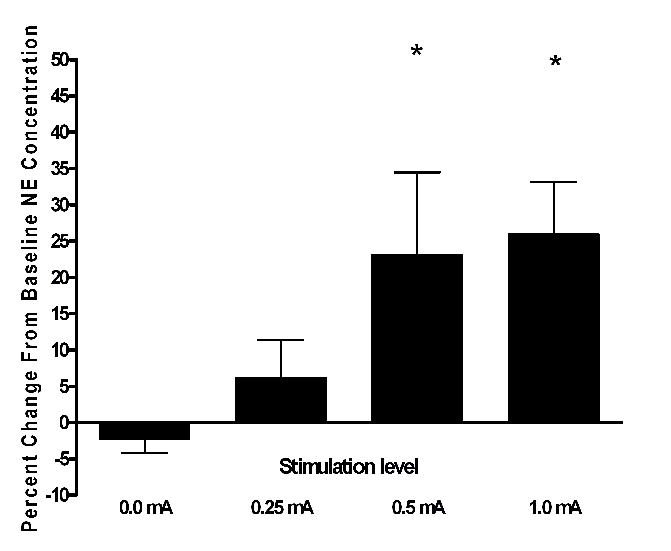
Effect of various intensities of VNS on extracellular NE in the hippocampus of freely moving rats, shown as percent change from baseline levels. The 0.5 and 1.0mA differed significantly from basal levels (* p<0.05, N= 12). Baseline NE concentration in dialysate from hippocampus averaged 0.096 ± 0.007pg/μL.
In the cortex the 0.0, 0.25, 0.5, and 1.0 mA levels of VNS produced percent changes in extracellular NE concentration of 6.27, −0.026, 10.29, and 39.0, respectively, when compared to baseline (Fig. 2). These percent changes in cortical NE concentrations varied significantly within subjects on the basis of level of stimulation [F(3,45) =16.32, p<0.016]. Pairwise comparisons yielded significant differences between animals receiving sham stimulation (0.0 mA) and those that received 1.0 mA (p<0.001) VNS.
Figure 2.
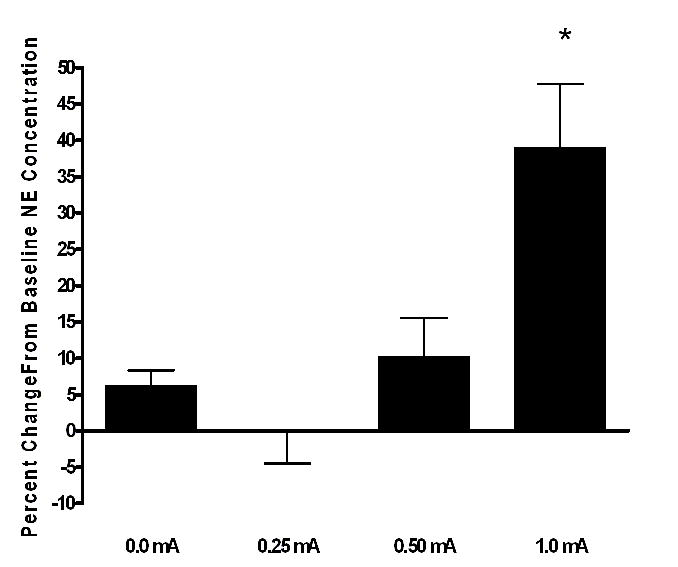
Effect of various intensities of VNS on extracellular NE in the cortex of freely moving rats, shown as percent change from baseline levels. The 1.0mA differed significantly from basal levels (* p<0.001, N= 8). Baseline NE concentration in dialysate from cerebral cortex averaged 0.102 ± 0.012 pg/μL.
Examination of the effect of 1.0 mA VNS on NE concentration in samples collected from cortical probes that were both ipsilateral and contralateral to the stimulated nerve revealed an increase in NE on both sides that was not significantly different (Fig. 3). The increases in NE concentrations were also found to be transient with stimulus-induced elevations essentially returning to baseline in the inter-stimulus period. An example of the transient nature of the VNS-induced increase in NE concentration in the hippocampus and cortex is depicted in Figure 4. Finally, a pairwise comparison showed that VNS had no significant effect (alpha of 0.05) on inter-stimulation baseline NE concentrations compared to initial baselines concentrations at any of the levels of VNS tested.
Figure 3.
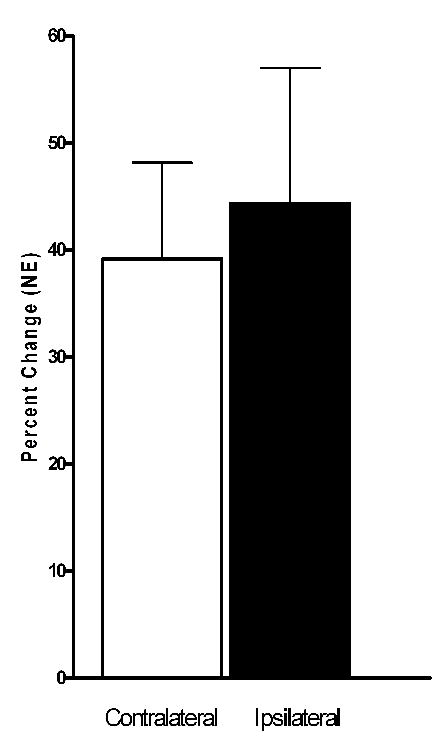
Increased extracellular NE concentrations in ipsilateral and contralateral cerebral cortex following left vagus nerve stimulation. These increases in extracellular NE did not differ significantly on the basis of hemisphere (p=0.49, N=6). Average baseline NE concentration in ipsilateral dialysate was 0.135 ± 0.012 pg/μL and in the contralateral dialysate was 0.095 ± 0.017 pg/μL.
Figure 4.
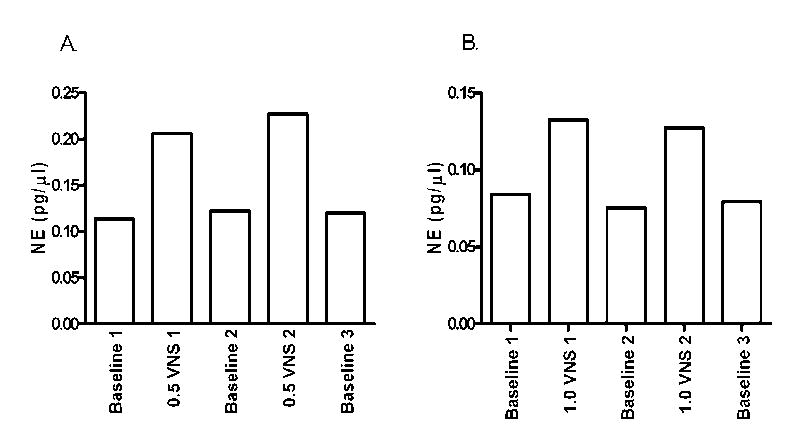
Panel A.: Changes in extracellular NE concentration in the hippocampus of one rat during alternating 1h periods of dialysate collection for baseline (stimulus off) and stimulation (VNS at 0.5 mA). Panel B: Change in extracellular NE concentration in the cortex of a rat during 1h periods of dialysate collection for baseline (stimulus off) and stimulation (VNS at 1.0 mA). Depicts actual concentration of NE (pg/μL) measured in each sample rather than percent change. Note that the NE concentration returns to baseline when vagus nerve is not being stimulated.
Discussion
This is the first demonstration of a VNS-induced increase in extracellular NE concentration in structures receiving noradrenergic innervation exclusively from the LC. Thus, it provides more direct evidence for a noradrenergic link as a potential mechanism of action of VNS in the modulation of learning and memory, recovery of function following CNS injury, supression of seizures, and improved affect in depression.
The VNS-induced increase in the concentration of extracellular NE following the 1.0 mA level of stimulation in both the hippocampus and cortex supports the hypothesis that NE contributes to the antiepileptic effects of VNS. Similarly, this level of stimulation has been used in rodent models of depression (Krahl et al., 2004) and may account for the mood elevating effects found with VNS treatment. The bilateral increase in extracellular NE following VNS was expected because it is consistent with the bilateral innervation of the NTS from each vagal nerve (Henry, 2002). The transient nature of the increase in extracellular NE following VNS is of interest in light of the capacity of a single stimulus train (30 sec) to improve memory in rats 24 h later. However, NE is known to facilitate long term potentiation (Stanton & Sarver, 1987), which is associated with changes in intracellular signaling some of which lead to changes in gene expression. Thus, prolonged changes in synaptic transmission resulting from transient increases in NE are not entirely unexpected.
When animals received the 0.5 mA VNS, the intensity that has been shown to improve functional recovery following CNS injury (Smith et al., 2005), an increase in hippocampal NE concentration of 23% was observed (p<0.05), but no parallel increase was seen in the cortex. While the basis of the difference in cortical and hippocampal responsiveness to VNS is unclear, it is consistent with previous findings suggesting that VNS has intensity dependent effects on cortical EEG and on learning and memory. This finding provides additional evidence that LC function varies across various behavioral and arousal states and that it has the capacity to act selectively on CNS targets rather than in a simple global pattern. At the lowest level of VNS tested (0.25 mA), which was slightly below the level recently shown to increase LC firing rates (0.3 mA) (Groves et al., 2005), we failed to observe a significant change in NE concentration in either the hippocampus or cortex. However, the single unit electrophysiological recording study by Groves et al (2005) did not investigate any other stimulus intensities. Thus, it is uncertain whether any increase in NE concentrations would be expected at the 0.25 mA intensity.
It is unlikely that the present findings were influenced by the cardiovascular effects (e.g. bradycardia) of VNS for at least three reasons. First, the animals received only left vagus nerve stimulation; and the vagal innervation of the heart is asymmetric, with the left vagus innervation being fairly sparse and being directed primarily to the atrioventricular node. In contrast, the right vagus nerve innervates the sinoatrial node (main pacemaker) and the atria (Saper et al., 1990). Thus, it is not surprising to find that left-sided VNS has little or no effect on heart rate in epilepsy patients receiving this therapy (Ramsay et al., 1994; Uthmann et al, 1993). Second, previous studies from our laboratory have shown that the behavioral effects of VNS persist even when downstream nerve fibers are anesthetized with lidocaine, blocking the efferent organ innervation (Clark et al., 1998). Third, the effects of VNS on NE release in the amygdala are still present when peripheral effects of VNS are blocked by methyl atropine (Hassert et al., 2003), providing further evidence of a centrally mediated effect of VNS that is independent of peripheral changes.
The pattern of response to VNS observed in the present study differs substantially from that reported by Hassert et al. (Hassert et al., 2004). In that study, a single stimulus train of 0.4 mA intensity lasting 30 sec was found to increase the concentration of extracellular NE in the amygdala by greater than 100% which remained elevated for at least two hours after termination of stimulation. In the present study, the increase in extracellular NE following VNS was quite transient, not significantly outlasting the period of stimulation (Figure 3). The reason for the disparity in findings between the present study and that of Hassert et al. (Hassert et al., 2004) is not known. However, at least two differences between that study and the present one may account for the observed differences. First, in addition to innervation from the LC, the basal lateral amygdala receives significant noradrenergic innervation from the lateral tegmental noradrenergic system, which may respond differently than the LC to VNS. Second, in the study by Hassert and co-workers.(Hassert et al., 2004), a single stimulus train was delivered, whereas in the present study multiple stimulations were delivered. Whether this could have contributed to prolonged versus transient effects on extracellular NE is not known.
To date relatively few studies investigating the effects of various VNS parameters have been conducted (Woodbury and Woodbury 1990; Zabara 1992; Clark et al., 1995; Clark et al., 1998; Clark et al., 1999). While stimulation parameters associated with the acute suppression of seizures and the enhancement of memory have been examined, there is little information about the optimal stimulation conditions for other effects of VNS such as facilitation of recovery of function or antidepressant effects. Moreover, there is evidence suggesting VNS effects change over time. For example, in patients receiving VNS for the treatment of epilepsy, the anticonvulsant effects of VNS continued to gain effectiveness beyond the initial 90 days of stimulation (DeGiorgio et al., 2000).
In conclusion, left VNS produced an intensity-dependent increase in NE concentrations in the hippocampus, reaching statistical significance at the 0.5 mA and 1.0 mA levels, but only the 1.0 mA stimulus produced a significant increase in NE concentration in the cortex. The VNS-induced increase in NE seen both in cortex and hippocampus was transient, returning to baseline in the inter-stimulus period. Moreover, VNS did not alter NE concentration during the inter-stimulation baseline periods. The present findings demonstrate that VNS increases NE output in the hippocampus and cortex at stimulation levels known to have a therapeutic benefit.
Experimental Procedure
Male Long Evans Hooded rats weighing 375–500 g at the time of surgery were anesthetized with chloral hydrate (400 mg/kg; IP) and given 100,000 units procaine penicillin-G IM. They were then implanted with bipolar stimulating electrodes on the left vagus nerve at the cervical level as described elsewhere [Smith et al., 2005)], and with CMA- 12 microdialysis guide cannulas located in the hippocampus and cortex. The skull was exposed with a midline incision extending from approximately 10 mm anterior to bregma caudal to the occipital pole. The skin was retracted and the surface of the skull cleared of all tissue. Two small machine screws (1.5 × 3.5 mm) were placed in the skull anterior to bregma and one adjacent to lambda to aid in the attachment of the probes with dental acrylic. With the incisor bar set at +5, guide cannulas for cortical probes were stereotaxically placed using the following coordinates: 0.6 mm rostral from bregma, and 6 mm lateral from the sagittal suture, and 0.2 mm ventral from the surface of the dura. Hippocampal guide cannulas were placed 3.8 mm caudal to bregma, 5.0 mm lateral to the sagittal suture, and 2.5 mm ventrally from the dura. In order to control for potential differences relative to the stimulated nerve, guide implants were alternated between hemispheres for each structure. Following cannula placement, the area surrounding the guides was covered with Gel foam®, the electrode wires were attached to a connector (Plastics One, Inc.) and dental acrylic was applied to affix the guide cannulas and electrode connector. The rats were then returned to their home cages for recovery.
Microdialysis probe implantation
In the afternoon prior to the first day of testing, 4–7 days following implantation of the guide cannulas, the rats were given 5 mg/kg diazepam for sedation and as an anticonvulsant (insertion of the hippocampal probe uniformly induced facial clonus), placed in Rat-turn™ chambers (BAS), connected to the VNS stimulator, and the dummy probes (stylets) were replaced with CMA-12 (2 mm) microdialysis probes. Artificial cerebral spinal fluid (aCSF), consisting of 150 mM NaCl, 3 mM KCl, 1.7 mM CaCl2·2H20, and 0.9 mM MgCl2·6H20 was perfused through the probes overnight at the rate of 0.2 μl/min. At least one hour prior to sample collection, aCSF flow rate was increased to 1.0 μL/min. Using a microliter infusion pump (CMA-102 pump) aCSF was perfused through the probes at the rate of 1 μL/min and 60 μL dialysis samples were collected in vials containing 30 μL oxalic acid or 30μL of perchloric acid as a preservative using a refrigerated fraction collector. The dialysate samples were stored at 4° C pending assay (always within seven days). We have found that samples stored under these conditions for 7 days show a small, albeit non-significant, decline in NE concentration (p=−.0626; t=2.283; df=6; n=7, paired t-test; day 1 vs day 7). However, because our data are expressed as percent change from baseline and because all samples from the same rat were stored identically, the sample storage would not have affected the percent changes.
Vagus Nerve Stimulation
Four vagus nerve stimulation intensities were tested: 0.0 mA (sham), 0.25 mA, 0.5 mA, and 1.0 mA all with a pulse width of 500 μS; a frequency of 20 Hz; duration of 30 seconds. Each experimental animal experienced five one-hour collection periods distributed as follows: 1) Baseline one, 2) stimulation one, 3) baseline two, 4) stimulation two, and 5) baseline three. Stimulation of the vagus nerve was administered using a Cyberonics prosthetics device programmed to deliver the specified stimulus intensity. Stimulation was delivered every 10 minutes during each one-hour stimulation period. Percent change in NE concentration was calculated by comparing the first baseline with the first stimulation and the second baseline and second stimulation for each stimulation intensity (NE concentration following stimulation minus baseline value divided by baseline and multiplied by 100). In order to determine if VNS altered NE concentrations between the inter-stimulation baselines, baseline two and three were compared to the first baseline. In order to control for sequence effect among the various stimulus intensities, the order of delivery of the four stimulus intensities were randomized with two levels of stimulation completed on the first day and two levels completed on the second day of stimulation. Thus, microdialysis samples were collected for a total of 20 h over two days at four different intensities. Immediately following the final collection vagus nerve electrode impedance was tested to insure adequate function. All electrode impedance values were < 7.0 K ohm.
Norepinephrine assay
Following collection, the microdialysis samples were assayed by high-performance liquid chromatography in combination with electrochemical detection (HPLC-ECD). Samples (25 μl) were injected by an ESA Model 542 autosampler into a 3.0 μM C-18 reverse phase analytical column (ESA MD-150 × 3.2). The mobile phase containing 75.0 mM lithium acetate dihydrate, 4.0 mM 1-heptane sulfonic acid, 100.0 μM EDTA, and 7.8% methanol ( pH of 4.7) was delivered by an ESA model 582 solvent delivery module at a rate of 0.6 ml/min. Electrochemical detection was carried out by an ESA Coulochem III detector. Analysis of sample content was performed using EZCHROMelite software (Scientific Software Inc). NE values were quantified by comparison of peak heights with standards. Detection threshold for NE was 1.7 pg on column.
Histological verification
Microdialysis probe positions were verified using 40 μM frozen sections stained with thionin and viewed on a Nikon profile projector by an investigator blinded to the microdialysis data. Only animals with probes located completely in the cortex/hippocampus were included in the data analysis. Photographs depicting the acceptable probe placement are shown in Figure 4.
Statistical analysis
Statistical analysis of changes in NE concentrations by stimulation group was performed with one-way repeated measures ANOVA with LSD adjustments for multiple comparisons of each stimulation group to the sham stimulation group. Statistical analysis to determine changes in baseline concentration of NE within each intensity was conducted using repeated measures ANOVA with LSD adjustments for multiple comparisons. A significance level of p < 0.05 was used for all statistical test conducted.
Figure 5.
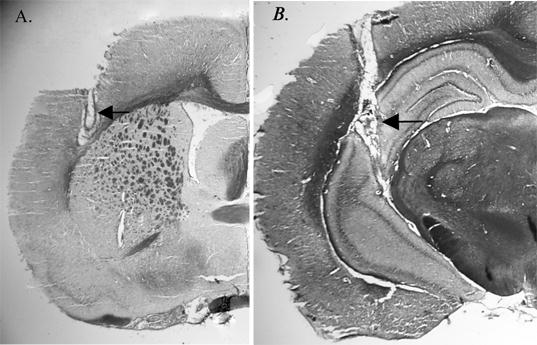
Representative histological sections showing location of microdialysis probes in: (A) cortex and (B) hippocampus. Arrows indicate location of dialysis membrane.
Acknowledgments
This work was supported by NIH grant RO1 NS 41551 and by Cyberonics, Inc. The authors are grateful for the excellent surgical skills of Arlene Tan. Thanks are also due to Soumya Banerjee assistance with surgeries, Maureen Doran for the histology, and Luke Sherrill for his electronic skills.
References
- Amar AP, Levy ML, Mc Comb JG, Apuzzo ML. Vagus nerve stimulation for control of intractable seizures in childhood. Pediatric Neurosurg. 2001;34:218–223. doi: 10.1159/000056023. [DOI] [PubMed] [Google Scholar]
- Armitage R, Husain M, Hoffman R, Rush AJ. The effects of vagus nerve stimulation on sleep EEG in depression: a preliminary report. J Psychosom Res. 2003;54:475–482. doi: 10.1016/s0022-3999(02)00476-2. [DOI] [PubMed] [Google Scholar]
- Ben-Menachem E. Modern management of epilepsy: Vagus nerve stimulation. Baillieres Clin Neurol. 1996;5:841–848. [PubMed] [Google Scholar]
- Ben-Menachem E. Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurol. 2002;1:477–482. doi: 10.1016/s1474-4422(02)00220-x. [DOI] [PubMed] [Google Scholar]
- Bolwig TG. Putative common pathways in therapeutic brain stimulation for affective disorders. CNS Spectr. 2003;8:490–495. doi: 10.1017/s1092852900018964. [DOI] [PubMed] [Google Scholar]
- Boon P, Vonck K, de Reuck J, Caemaert J. Vagus nerve stimulation for refractory epilepsy. Seizure. 2002;11(Suppl A):448–455. [PubMed] [Google Scholar]
- Boyeson MG. Effects of fluoxetine and maprotiline on functional recovery in poststroke hemiplegic patients undergoing rehabilitation therapy. Stroke. 1996;27:1211–1214. doi: 10.1161/01.str.27.7.1211. [DOI] [PubMed] [Google Scholar]
- Boyeson MG, Feeney DM. Intraventricular norepinephrine facilitates motor recovery following sensorimotor cortex injury. Pharmacol Biochem behav. 1990;35:497–501. doi: 10.1016/0091-3057(90)90279-q. [DOI] [PubMed] [Google Scholar]
- Boyeson MG, Krobert KA. Cerebellar norepinephrine infusions facilitate recovery after sensorimotor cortex injury. Brain res bull. 1992;29:435–439. doi: 10.1016/0361-9230(92)90080-h. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Friehs GM, Price LH. Cervical vagus nerve stimulation for treatment-resistant depression. Neurosurg Clin N Am. 2003;14:275–282. doi: 10.1016/s1042-3680(02)00121-3. [DOI] [PubMed] [Google Scholar]
- Chavel SM, Westerveld M, Spencer S. Long-term outcome of vagus nerve stimulation for refractory partial epilepsy. Epilepsy Behav. 2003;4:302–309. doi: 10.1016/s1525-5050(03)00109-4. [DOI] [PubMed] [Google Scholar]
- Clark KB, Krahl SE, Smith DC, Jensen RA. Post-training unilateral vagal stimulation enhances retention performance in the rat. Neurobiol Learn Mem. 1995;63:213–216. doi: 10.1006/nlme.1995.1024. [DOI] [PubMed] [Google Scholar]
- Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci. 1999;2:94–98. doi: 10.1038/4600. [DOI] [PubMed] [Google Scholar]
- Clark KB, Smith DC, Hassert DL, Browning RA, Naritoku DK, Jensen RA. Posttraining electrical stimulation of vagal afferents with concomitant vagal efferent inactivation enhances memory storage processes in the rat. Neurobiol Learn Mem. 1998;70:364–373. doi: 10.1006/nlme.1998.3863. [DOI] [PubMed] [Google Scholar]
- Crumrine PK. Vagal nerve stimulation in children. Semin Pediatr Neurol. 2000;7:216–223. doi: 10.1053/spen.2000.9218. [DOI] [PubMed] [Google Scholar]
- DeGiorgio CM, Schachter SC, Handforth A, Salinsky M, Thompson J, Uthman B, Reed R, Collins S, Tecoma E, Morris GL, Vaughn B, Naritoku DK, Henry T, Labar D, Gilmartin R, Labiner D, Osorio I, Ristanovic R, Jones J, Murphy J, Ney G, Wheless J, Lewis P, Heck C. Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia. 2000;41:1195–1200. doi: 10.1111/j.1528-1157.2000.tb00325.x. [DOI] [PubMed] [Google Scholar]
- Dunn-Meynell A, Pan S, Levin BE. Focal traumatic brain injury causes widespread reductions in rat brain norepinephrine turnover from 6 to 24 h. Brain Res. 1994;660:88–95. doi: 10.1016/0006-8993(94)90842-7. [DOI] [PubMed] [Google Scholar]
- Elger G, Hoppe C, Falkai P, Rush AJ, Elger CE. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res. 2000;42:203–210. doi: 10.1016/s0920-1211(00)00181-9. [DOI] [PubMed] [Google Scholar]
- Feeney DM, Gonzales A, Law WA. Amphetamine restores locomotor function after motor cortex injury in the rat. Proc West Pharmacol Soc. 1981;24:15–17. [PubMed] [Google Scholar]
- Feeney DM, Gonzalez A, Law WA. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217:855–857. doi: 10.1126/science.7100929. [DOI] [PubMed] [Google Scholar]
- Feeney DM, Hovda DA. Amphetamine and apomorphine restore tactile placing after motor cortex injury in the cat. Psychopharmacology (Berl) 1983;79:67–71. doi: 10.1007/BF00433018. [DOI] [PubMed] [Google Scholar]
- Feeney DM, Weisend MP, Kline AE. Noradrenergic pharmacotherapy, intracerebral infusion and adrenal transplantation promote functional recovery after cortical damage. J Neural Transplant Plast. 1993;4:199–213. doi: 10.1155/NP.1993.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Guardiola A, Martinez A, Valdes-Cruz A, Magdaleno-Madrigal VM, Martinez D, Fernandez-Mas R. Vagus nerve prolonged stimulation in cats: effects on epileptogenesis (amygdala electrical kindling): behavioral and electrographic changes. Epilepsia. 1999;40:822–829. doi: 10.1111/j.1528-1157.1999.tb00787.x. [DOI] [PubMed] [Google Scholar]
- George MS, Sackeim HA, Marangell LB, Husain MM, Nahas Z, Lisanby SH, Ballenger JC, Rush AJ. Vagus nerve stimulation. A potential therapy for resistant depression? Psychiatr Clin North Am. 2000;23:757–783. doi: 10.1016/s0193-953x(05)70196-9. [DOI] [PubMed] [Google Scholar]
- George MS, Rush AJ, Marangell LB, Sackeim HA, Brannan SK, Davis SM, Howland R, Kling MA, Moreno F, Rittberg B, Dunner D, Schwartz T, Carpenter L, Burke M, Ninan P, Goodnick P. A one-year comparison of vagus nerve stimulation with treatment as usual for treatment-resistant depression. Biol. Psychiatry. 2005;58:364–373. doi: 10.1016/j.biopsych.2005.07.028. [DOI] [PubMed] [Google Scholar]
- Goldstein LB, Davis JN. Clonidine impairs recovery of beam-walking after a sensorimotor cortex lesion in the rat. Brain research. 1990;508:305–309. doi: 10.1016/0006-8993(90)90413-6. [DOI] [PubMed] [Google Scholar]
- Groves DA, Bowman EM, Brown VJ. Recordings from the rat locus coeruleus during acute vagal nerve stimulation in the anaesthetised rat. Neurosci lett. 2005;379:174–179. doi: 10.1016/j.neulet.2004.12.055. [DOI] [PubMed] [Google Scholar]
- Handforth A, DeGiorgio CM, Schachter SC, Uthman BM, Naritoku DK, Tecoma ES, Henry TR, Collins SD, Vaughn BV, Gilmartin RC, Labar DR, Morris GL, 3rd, Salinsky MC, Osorio I, Ristanovic RK, Labiner DM, Jones JC, Murphy JV, Ney GC, Wheless JW. Vagus nerve stimulation therapy for partial -onset seizures. A randomized active-control trial. Neurology. 1998;51:48–55. doi: 10.1212/wnl.51.1.48. [DOI] [PubMed] [Google Scholar]
- Harden CL, Pulver MC, Ravdin LD. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav. 2000;1:93–99. doi: 10.1006/ebeh.2000.0046. [DOI] [PubMed] [Google Scholar]
- Hassert DL, Miyashita T, Williams CL. The effects of peripheral vagal nerve stimulation at a memory-modulating intensity on norepinephrine output in the basolateral amygdala. Behav Neurosci. 2004;118:79–88. doi: 10.1037/0735-7044.118.1.79. [DOI] [PubMed] [Google Scholar]
- Henry TR, Bakay RA, Pennell PB, Epstein CM, Votaw JR. Brain blood-flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: II. prolonged effects at high and low levels of stimulation. Epilepsia. 2004;45:1064–1070. doi: 10.1111/j.0013-9580.2004.03104.x. [DOI] [PubMed] [Google Scholar]
- Henry TR. Therapeutic mechanisms of vagus nerve stimulation. Neurology. 2002;59(Suppl 4):S3–S14. doi: 10.1212/wnl.59.6_suppl_4.s3. [DOI] [PubMed] [Google Scholar]
- Henry TR, Bakay RA, Votaw JR, Pennell PB, Epstein CM, FaberTLGrafton ST. Brain blood flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: I. Acute effects at high and low levels of stimulation. Epilepsia. 1998;39:983–990. doi: 10.1111/j.1528-1157.1998.tb01448.x. [DOI] [PubMed] [Google Scholar]
- Holmes MD, Silbergeld DL, Drouhard D, Wilensky AJ, Ojemann LM. Effect of vagus nerve stimulation on adults with pharmacoresistant generalized epilepsy syndromes. Seizure. 2004;13:340–345. doi: 10.1016/j.seizure.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Hoppe C, Helmstaedter C, Scherrmann J, Elger CE. Self-Reported Mood Changes following 6 Months of Vagus Nerve Stimulation in Epilepsy Patients. Epilepsy Behav. 2001;2:335–342. doi: 10.1006/ebeh.2001.0194. [DOI] [PubMed] [Google Scholar]
- Hovda DA, Sutton RL, Feeney DM. Recovery of tactile placing after visual cortex ablation in cat: a behavioral and metabolic study of diaschisis. Exp Neurol. 1987;97:391–402. doi: 10.1016/0014-4886(87)90099-9. [DOI] [PubMed] [Google Scholar]
- Hovda DA, Sutton RL, Feeney DM. Amphetamine-induced recovery of visual cliff performance after bilateral visual cortex ablation in cats: measurements of depth perception thresholds. Behav Neurosci. 1989;103:574–584. doi: 10.1037//0735-7044.103.3.574. [DOI] [PubMed] [Google Scholar]
- Huf RL, Mamelak A, Kneedy-Cayem K. Vagus nerve stimulation therapy: 2-year prospective open-label study of 40 subjects with refractory epilepsy and low IQ who are living in long-term care facilities. Epilepsy Behavior. 2005;6:417–423. doi: 10.1016/j.yebeh.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Hui AC, Lam JM, Wong KS, Kay R, Poon WS. Vagus nerve stimulation for refractory epilepsy: long term efficacy and side-effects. Chin Med J (Engl) 2004;117:58–61. [PubMed] [Google Scholar]
- Kikuchi K, Nishino K, Ohyu H. L-DOPS-Accelerated recovery of locomotor function in rats subjected to sensorimotor cortex ablation injury: pharmacobehavioral studies. Tohoku J Exp Med. 1999;188:203–215. doi: 10.1620/tjem.188.203. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Nishino K, Ohyu H. Increasing CNS norepinephrine levels by the precursor L-DOPS facilitate beam-walking recovery after sensorimotor cortex ablation in rats. Brain Res. 2000;860:130–135. doi: 10.1016/s0006-8993(00)02034-5. [DOI] [PubMed] [Google Scholar]
- Kossoff EH, Pyzik PL. Improvement in alertness and behavior in children treated with combination topiramate and vagus nerve stimulation. Epilepsy Behav. 2004;5:256–259. doi: 10.1016/j.yebeh.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Koszewski W, Bacia T, Rysz A. Vagus nerve stimulation (VNS) in the treatment of drug-resistant epilepsy. A 4-year follow-up evaluation of VNS treatment efficacy. Neurol Neurochir Pol. 2003;37:573–586. [PubMed] [Google Scholar]
- Krahl SE, Clark KB, Smith DC, Browning RA. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia. 1998;39:709–714. doi: 10.1111/j.1528-1157.1998.tb01155.x. [DOI] [PubMed] [Google Scholar]
- Krahl SE, Senanayake SS, Handforth A. Right-sided vagus nerve stimulation reduces generalized seizure severity in rats as effectively as left-sided. Epilepsy Res. 2003;56:1–4. doi: 10.1016/s0920-1211(03)00122-0. [DOI] [PubMed] [Google Scholar]
- Krahl SE, Senanayake SS, Pekary AE, Sattin A. Vagus nerve stimulation (VNS) is effective in a rat model of antidepressant action. J Psychiatr Res. 2004;38:237–240. doi: 10.1016/j.jpsychires.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Krobert KA, Sutton RL, Feeny DM. Spontaneous and amphetamine-evoked release of cerebellar noradrenaline after sensorimotor cortex contusion: an in vivo microdialysis study in the awake rat. J Neurochem. 1994;62:2233–2240. doi: 10.1046/j.1471-4159.1994.62062233.x. [DOI] [PubMed] [Google Scholar]
- Leonard BE. The role of noradrenaline in depression: a review. J Psychopharmacol. 1997;11:S39–47. [PubMed] [Google Scholar]
- Levin BE, Brown KL, Pawer G, Dunn-Meynell A. Widespread and lateralization effects of acute traumatic brain injury on norepinephrine turnover in the rat brain. Brain Res. 1995;674:307–313. doi: 10.1016/0006-8993(95)00032-l. [DOI] [PubMed] [Google Scholar]
- Loughlin SE, Foote KL, Fallon JH. Locus coeruleus projections to cortex: topography, morphology and collateralization. Brain Res Bull. 1982;9:287–294. doi: 10.1016/0361-9230(82)90142-3. [DOI] [PubMed] [Google Scholar]
- Loughlin SE, Foote SL, Grzanna R. Efferent projections of nucleus locus coeruleus: morphologic subpopulations have different efferent targets. Neuroscience. 1986;18:307–319. doi: 10.1016/0306-4522(86)90156-9. [DOI] [PubMed] [Google Scholar]
- Loy R, Koziell DA, Lindsey JD, Moore RY. Noradrenergic innervation of the adult rat hippocampal formation. J Comp Neurol. 1980;189:699–710. doi: 10.1002/cne.901890406. [DOI] [PubMed] [Google Scholar]
- Macritchie KA, Young AH. Emerging targets for the treatment of depressive disorder. Expert Opin Ther Targets. 2001;5:601–612. doi: 10.1517/14728222.5.5.601. [DOI] [PubMed] [Google Scholar]
- Mc Gaugh JL. Memory-accenting of consolidation. Science. 2000;287:248–251. [Google Scholar]
- Marangell LB, Rush AJ, George MS, Sackeim HA, Johnson CR, Husain MM, Nahas Z, Lisanby SH. Vagus nerve stimulation (VNS) for major depressive episodes: one year outcomes. Biol Psychiatry. 2002;51:280–287. doi: 10.1016/s0006-3223(01)01343-9. [DOI] [PubMed] [Google Scholar]
- McIntosh TK, Yu T, Gennarelli TA. Alterations in regional brain catecholamine concentrations after experimental brain injury in the rat. J Neurochem. 1994;63:1426–1433. doi: 10.1046/j.1471-4159.1994.63041426.x. [DOI] [PubMed] [Google Scholar]
- Meyer P, Horel J, Meyer D. Effects of dl-amphetamine upon placing responses in neodecorticate cats. J Comp Physiol Psych. 1963;56:402–404. doi: 10.1037/h0049297. [DOI] [PubMed] [Google Scholar]
- Michael JE, Wegener K, Barnes DW. Vagus nerve stimulation for intractable seizures: one year follow-up. J Neurosci Nurs. 1993;25:362–6. doi: 10.1097/01376517-199312000-00007. [DOI] [PubMed] [Google Scholar]
- Moore RY, Card JP. Noradrenaline-containing neuron systems. In: Bjèorklund A, Hèokfelt T, Kuhar MJ, editors. Handbook of Chemical Neuroanatomy: Classical Transmitters in the CNS Part I. Elsievier; 1984. [Google Scholar]
- Morris GL, Mueller WM. Long-term treatment with vagus nerve stimulation in patients with refractory epilepsy. The Vagus Nerve Stimulation Study Group E01-E05. Neurology. 1999;53:1731–1735. doi: 10.1212/wnl.53.8.1731. [DOI] [PubMed] [Google Scholar]
- Nagarajan L, Walsh P, Gregory P, Lee M. VNS therapy in clinical practice in children with refractory epilepsy. Acta Neurol Scand. 2002;105:13–17. doi: 10.1034/j.1600-0404.2002.00129.x. [DOI] [PubMed] [Google Scholar]
- Naritoku DK, Terry WJ, Helfert RH. Regional induction of fos immunoreactivity in the brain by anticonvulsant stimulation of the vagus nerve. Epilepsy Res. 1995;22:53–62. doi: 10.1016/0920-1211(95)00035-9. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Jensen RA. Beta-adrenergic receptor agonist antihypertensive medications impair arousal-induced modulation of working memory in elderly humans. Behav Neural Biol. 1995;62:190–200. doi: 10.1016/s0163-1047(05)80017-2. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Radtke RC, Jensen RA. Arousal-induced modulation of memory storage processes in humans. Neurobiology of Learning and Memory. 1996;66:133–142. doi: 10.1006/nlme.1996.0054. [DOI] [PubMed] [Google Scholar]
- Prasad MR, Tzigaret CM, Smith D, Soares H, McIntosh TK. Decreased alpha 1-adrenergic receptors after experimental brain injury. J Neurotrauma. 1992;9:269–279. doi: 10.1089/neu.1992.9.269. [DOI] [PubMed] [Google Scholar]
- Queen SA, Chen MJ, Feeney DM. d-Amphetamine attenuates decreased cerebral glucose utilization after unilateral sensorimotor cortex contusion in rats. Brain Res. 1997;777:42–50. doi: 10.1016/s0006-8993(97)00717-8. [DOI] [PubMed] [Google Scholar]
- Ramsey RE, Uthman BM, Augustinsson LE, Upton ARM, Naritoku D, Willis J, Treig T, Barolat G, Wernicke JF. 1994. Vagus nerve stimulation for treatment of partial seizures. 2. Safety, side effects and tolerability. Epilepsia. 1994;35:627–636. doi: 10.1111/j.1528-1157.1994.tb02483.x. [DOI] [PubMed] [Google Scholar]
- Rush AJ, George MS, Sackeim HA, Marangell LB, Husain MM, Giller C, Nahas Z, Haines S, Simpson RK, Jr, Goodman R. Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biol Psychiatry. 2000;47:276–286. doi: 10.1016/s0006-3223(99)00304-2. [DOI] [PubMed] [Google Scholar]
- Saper CB, Kibbe MR, Hurley KM, Spencer S, Holmes HR, Leahy KM, Needleman P. Brain natriuretic peptide-like immunoreactive innervation of the cardiovascular and cerebrovascular systems in the rat. Circ Res. 1990;67:1345–1354. doi: 10.1161/01.res.67.6.1345. [DOI] [PubMed] [Google Scholar]
- Sirven JI, Sperling M, Naritoku D, Schachter S, Labar D, Holmes M, Wilensky A, Cibula J, Labiner DM, Bergen D, Ristanovic R, Harvey J, Dasheiff R, Morris GL, O'Donovan CA, Ojemann L, Scales D, Nadkarni M, Richards B, Sanchez JD. Vagus nerve stimulation therapy for epilepsy in older adults. Neurology. 2000;54:1179–1182. doi: 10.1212/wnl.54.5.1179. [DOI] [PubMed] [Google Scholar]
- Smith DC, Modglin A, Roosevelt RW, Neese S, Jensen RA, Browning RA, Clough RW. Electrical stimulation of the vagus nerve enhances cognitive and motor recovery following moderate fluid percussion injury in the rat. J Neurotrauma. 2005;22:1485–1502. doi: 10.1089/neu.2005.22.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton PK, Sarver JM. Norepinephrine regulates long-term potentiation of both the population spike and dendritic EPSP in hippocampal dentate gyrus. Brain Res Bull. 1987;18:115–119. doi: 10.1016/0361-9230(87)90039-6. [DOI] [PubMed] [Google Scholar]
- Takaya M, Terry WJ, Naritoku DK. Vagus nerve stimulation induces a sustained anticonvulsant effect. Epilepsia. 1996;37:1111–1116. doi: 10.1111/j.1528-1157.1996.tb01033.x. [DOI] [PubMed] [Google Scholar]
- Takigawa M, Mogenson GJ. A study of inputs to antidromically identified neurons of the locus coeruleus. Brain Res. 1977;135:217–230. doi: 10.1016/0006-8993(77)91027-7. [DOI] [PubMed] [Google Scholar]
- Tanganelli P, Ferrero S, Colotto P, Regesta G. Vagus nerve stimulation for treatment of medically intractable seizures. Evaluation of long-term outcome. Clin Neurol Neurosurg. 2002;105:9–13. doi: 10.1016/s0303-8467(02)00018-5. [DOI] [PubMed] [Google Scholar]
- Topfer LA, Hailey D. Vagus nerve stimulation (VNS) for treatment-resistant depression. Issues Emerg Health Technol. 2001;25:1–4. [PubMed] [Google Scholar]
- Uthman BM, Wilder BJ, Penry JK, Dean C, Ramsay RE, Reid SA, Hammond EJ, Tarver WB, Wernicke JF. Treatment of epilepsy by stimulation of the vagus nerve. Neurology. 1993;43:1338–1345. doi: 10.1212/wnl.43.7.1338. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele, E. J., Peoples, J., Telegan, P., 1999. Efferent projections of the nucleus of the solitary tract to peri-locus coeruleus dendrites in rat brain: evidence for a monosynaptic pathway. J. Comp Neurol. 412, 410–428. [DOI] [PubMed] [Google Scholar]
- Wakai S, Kotagal P. Vagus nerve stimulation for children and adolescents with intractable epilepsies. Pediatr Int. 2001;43:61–65. doi: 10.1046/j.1442-200x.2001.01326.x. [DOI] [PubMed] [Google Scholar]
- Wheless JW, Baumgartner J. Vagus nerve stimulation therapy. Drugs Today (Barc) 2004;40:501–515. doi: 10.1358/dot.2004.40.6.850483. [DOI] [PubMed] [Google Scholar]
- Williams CL, McGaugh JL. Reversible lesions of the nucleus of the solitary tract attenuate the memory-modulating effects of posttraining epinephrine. Behav Neurosci. 1993;107:955–962. [PubMed] [Google Scholar]
- Woodbury DM, Woodbury JW. Effects of vagal stimulation on experimentally induced seizures in rats. Epilepsia. 1990;31(Suppl 2):S7–19. doi: 10.1111/j.1528-1157.1990.tb05852.x. [DOI] [PubMed] [Google Scholar]
- Zabara J. Inhibition of experimental seizures in canines by repetitive vagal stimulation. Epilepsia. 1992;33:1005–1012. doi: 10.1111/j.1528-1157.1992.tb01751.x. [DOI] [PubMed] [Google Scholar]


