Abstract
Hydrogen sulfide (H2S) is a naturally occurring gaseous transmitter, which may play important roles in normal physiology and disease. Here, we investigated the role of H2S in the organ injury caused by severe endotoxemia in the rat.
Male Wistar rats were subjected to acute endotoxemia (Escherichia coli lipopolysaccharide (LPS) 6 mg kg−1 intravenously (i.v.) for 6 h) and treated with vehicle (saline, 1 ml kg−1 i.v.) or DL-propargylglycine (PAG, 10–100 mg kg−1 i.v.), an inhibitor of the H2S-synthesizing enzyme cystathionine-γ-lyase (CSE). PAG was administered either 30 min prior to or 60 min after the induction of endotoxemia.
Endotoxemia resulted in circulatory failure (hypotension and tachycardia) and an increase in serum levels of alanine aminotransferase and aspartate aminotransferase (markers for hepatic injury), lipase (indicator of pancreatic injury) and creatine kinase (indicator of neuromuscular injury). In the liver, endotoxemia induced a significant increase in the myeloperoxidase (MPO) activity, and in the expression and activity of the H2S-synthesizing enzymes CSE and cystathionine-β-synthase.
Administration of PAG either prior to or after the injection of LPS dose-dependently reduced the hepatocellular, pancreatic and neuromuscular injury caused by endotoxemia, but not the circulatory failure. Pretreatment of rats with PAG abolished the LPS-induced increase in the MPO activity and in the formation of H2S and in the liver.
These findings support the view that an enhanced formation of H2S contributes to the pathophysiology of the organ injury in endotoxemia. We propose that inhibition of H2S synthesis may be a useful therapeutic strategy against the organ injury associated with sepsis and shock.
Keywords: Hydrogen sulfide, DL-propargylglycine, LPS, cystathionine-γ-lyase, CSE, rat
Introduction
Gaseous transmitters, such as nitric oxide and carbon monoxide (CO), play important roles both in physiology and in disease. In recent years, another naturally occurring gas, hydrogen sulfide (H2S), has been found to be of importance (Moore et al., 2003). Cystathionine-γ-lyase (CSE) and cystathionine-β-synthase (CBS) are key enzymes of the trans-sulfuration pathway, which interconverts L-methionine and L-cysteine, but can also use L-cysteine as an alternative substrate to form H2S. Both enzymes are pyridoxal phosphate dependent and are expressed in a range of mammalian cells and tissues. Although other enzymes can catalyze the production of H2S, CBS seems to be the main H2S-forming enzyme in the central nervous system, whereas CSE is the main enzyme forming H2S in the cardiovascular system (Moore et al., 2003).
H2S dilates blood vessels in vivo and in vitro probably by opening vascular smooth muscle K+-ATP channels (Zhao et al., 2001; Moore et al., 2003; Cheng et al., 2004). Some evidence has also been published of the ability of the K+-ATP channel blocker glibenclamide to inhibit the hypotensive response to a H2S donor sodium hydrogen sulfide (NaHS) (Geng et al., 2004). However, the role of H2S in the regulation of blood pressure or vascular perfusion remains unclear. In a rat model of hypoxic pulmonary hypertension, a reduction in the expression and activity of CSE was associated with a decrease in plasma H2S concentration in the lung tissue (Chunyu et al., 2003). Notably, parenteral H2S administration in these animals opposed the rise in pulmonary arterial pressure and partially prevented the pulmonary vascular remodelling. Therefore, H2S deficiency may contribute to the pathophysiology of this disease (Moore et al., 2003). It has also been found that exogenous supply of an inhibitor of H2S synthesis decreased the plasma H2S content and worsened hypoxic pulmonary hypertension (Qingyou et al., 2004). At the same time, plasma CO level and the expressions of hemioxygenase (HO)-1 protein and mRNA in pulmonary arteries were decreased. These results suggested that H2S could play a regulatory role in the pathogenesis of hypoxic pulmonary hypertension through upregulating CO/HO pathway. The possibility that excess H2S contributes to the hypotension associated with either septic (cecal ligation and puncture) or endotoxic shock in the rat has been suggested (Hui et al., 2003). In that study, the systemic inflammation resulted in increased H2S concentrations in the aorta, pulmonary, mesenteric and tail arteries when compared with controls, suggesting that overproduction of vasodilatory H2S may play a role in the vascular consequences of septic and endotoxic shock. In hemorrhagic shock, inhibitors of H2S synthesis were recently shown to reduce partially the hypotensive response associated with hemorrhage (Mok et al., 2004). Hemorrhage also increased the synthesis of H2S in the liver (Mok et al., 2004).
The role of H2S in inflammation is beginning to emerge. A recent report demonstrated that endogenous H2S plays a role in the pathophysiology of cerulein-induced pancreatitis (Bhatia et al., 2005b). Severe acute pancreatitis was associated with lung injury characterized by neutrophil accumulation, which was attenuated by treatment with an irreversible inhibitor of H2S-synthesizing enzyme CSE, DL-propargylglycine (PAG) (Hosoki et al., 1997; Teague et al., 2002; Cheng et al., 2004; Mok et al., 2004). Another recent study provided evidence of the role of H2S in inflammation, by showing that inhibition of CSE reduced the hindpaw edema induced by carrageenan injection (Bhatia et al., 2005a). Hence, the present study was designed to investigate the effects of endogenous H2S on the organ injury caused by acute severe endotoxemia in the rat, by using PAG, an inhibitor of H2S-synthesizing enzyme CSE.
Methods
Surgical procedure and quantification of organ injury
This study was carried out on 54 male Wistar rats (Tuck, Rayleigh, Essex, U.K.) weighing 220–320 g, receiving a standard diet and water ad libitum. The investigation was performed in accordance with the Home Office Guidance on the Operation of the Animals (Scientific Procedures) Act 1986, published by HMSO, London. All animals were anesthetized with thiopentone sodium (Intraval®, 120 mg kg−1 intraperitoneally (i.p.)), and anesthesia was maintained by supplementary injections of thiopentone sodium (approximately 1–2 mg kg−1 h−1 intravenously (i.v.)) as required. The general surgical procedures were performed as described previously (Millar & Thiemermann, 2002). Upon completion of the surgical procedure, mean arterial pressure (MAP) and heart rate (HR) were allowed to stabilize for 15 min. At 6 h after the induction of endotoxemia, 1.5 ml of blood was collected into a serum gel S/1.3 tube (Sarstedt, Germany) from a catheter placed in the carotid artery, and values were examined as previously described for indices of liver injury (Baue, 1993; Hewett et al., 1993), pancreatic injury (Ruetten & Thiemermann, 1997) and neuromuscular injury (Ruetten et al., 1996). All serum samples were analyzed within 24 h by a contract laboratory for veterinary clinical chemistry (Vetlab Services, Sussex, U.K.).
Experimental design
Animals were assigned to five experimental groups:
Sham Control: Rats were treated with saline (vehicle for PAG, 1 ml kg−1 i.v., n=9) without causing endotoxemia (received saline rather than endotoxin).
Sham PAG: Rats were treated with PAG (50 mg kg−1 i.v., n=6) without causing endotoxemia. The dose of PAG was chosen based on recent report showing inhibition of H2S formation in the rat liver at this dose (Mok et al., 2004).
Lipopolysaccharide (LPS) Control: Rats were treated with saline (1 ml kg−1 i.v., n=9) at 30 min before they were subjected to endotoxemia: Escherichia coli LPS (6 mg kg−1 i.v., serotype 0127:B8) was given slowly over 10 min.
LPS PAG pretreatment with 10 mg kg−1(LPS PAG 10 Pre): Rats were treated with PAG 10 mg kg−1 i.v. (n=8) at 30 min before they were subjected to endotoxemia.
LPS PAG pretreatment with 50 mg kg−1(LPS PAG 50 Pre): Rats were treated with PAG 50 mg kg−1 i.v. (n=9) at 30 min before they were subjected to endotoxemia.
LPS PAG post-treatment with 50 mg kg−1(LPS PAG 50 Post): Rats were treated with PAG 50 mg kg−1 i.v. (n=9) at 60 min after they were subjected to endotoxemia.
LPS PAG pretreatment with 100 mg kg−1(LPS PAG 100 Pre): Rats were treated with PAG 100 mg kg−1 i.v. (n=4) at 30 min before they were subjected to endotoxemia.
Measurement of myeloperoxidase (MPO) activity
Neutrophil sequestration in the liver was quantified by measuring tissue MPO activity (Bhatia et al., 1998, 2000). Liver samples were thawed, homogenized in 20 mM phosphate buffer (pH 7.4), centrifuged (10,000 × g, 10 min, 4°C) and the resulting pellet resuspended in 50 mM phosphate buffer (pH 6.0) containing 0.5% (w v−1) hexadecyltrimethylammonium bromide (Sigma-Aldrich Corp., St Louis, MO, U.S.A.). The suspension was subjected to four cycles of freezing and thawing and further disrupted by sonication (40 s). The sample was then centrifuged (10,000 × g, 5 min, 4°C) and the supernatant used for the MPO assay. The reaction mixture consisted of the supernatant (50 μl), 1.6 mM tetramethylbenzidine (Sigma), 80 mM sodium phosphate buffer (pH 5.4) and 0.3 mM hydrogen peroxide (reagent volume: 50 μl). This mixture was incubated at 37°C for 110 s, the reaction terminated with 50 μl of 0.18 M H2SO4 and the absorbance measured at 450 nm. This absorbance was then corrected for the DNA content of the tissue sample and results are expressed as enzyme activity.
Assay of liver H2S synthesis
Liver H2S-synthesizing activity was determined essentially as described elsewhere (Stipanuk & Beck, 1982). Briefly, liver tissue from animals treated as above was thawed and homogenized (Ultra-Turrax) in 100 mM ice-cold potassium phosphate buffer (pH 7.4). Optimal (w v−1) ratios of 1 : 20 were determined from preliminary experiments. The reaction mixture (total volume, 500 μl) contained L-cysteine (10 mM; 20 μl), pyridoxal 5′-phosphate (2 mM; 20 μl), saline (30 μl) and tissue homogenate (430 μl). The reaction was performed in parafilmed eppendorf tubes and initiated by transferring the tubes from ice to a water bath at 37°C. In some experiments, the enzymatic reaction was stopped immediately by the addition of trichloroacetic acid (10% (w v−1), 250 μl) to denature protein prior to the addition of cysteine. After incubation for 30 min, zinc acetate (1% (w v−1), 250 μl) was added to trap evolved H2S followed by trichloroacetic acid (10% (w v−1), 250 μl). Subsequently, N,N-dimethyl-p-phenylenediamine sulfate (20 μM; 133 μl) in 7.2 M HCl and FeCl3 (30 μM; 133 μl) in 1.2 M HCl were added and the absorbance of the resulting solution (670 nm) measured 15 min thereafter using a 96-well microplate reader (Tecan Systems Inc., San Jose, CA, U.S.A.). The basal concentration of H2S was determined in incubates in which trichloroacetic acid was added at zero time (T=0) prior to the addition of cysteine and incubation (37°C, 30 min). At the end of this period, trichloroacetic acid (10% (w v−1), 250 μl) was added and H2S generated assayed spectrophotometrically as described above. All samples were assayed in duplicate. The H2S concentration of each sample was calculated against a calibration curve of NaHS (3.12–250 μM) and results are expressed as nmol H2S formed mg−1 protein (determined using the Bradford assay, Bio-Rad Ltd, Hercules, CA, U.S.A.).
Reverse transcription–polymerase chain reaction (RT–PCR) analysis of liver CSE and CBS mRNA
Expression of CSE and CBS in tissues was determined essentially as described previously (Mok et al., 2004). Briefly, liver tissue (100 mg) was homogenized in 1 ml ice-cold TRIzol reagent (Invitrogen, Carlsbad, CA, U.S.A.) using a Polytron homogenizer (Heidolph Ltd, Schwabach, Germany) and thereafter incubated for 10 min at room temperature. Samples were mixed with chloroform (0.2 ml), vigorously shaken and incubated at room temperature for 3 min followed by centrifugation (12,000 × g, 4°C, 15 min). The upper aqueous phase was transferred to an eppendorf tube and isopropanol (0.5 ml) added. After further incubation at room temperature (10 min), samples were recentrifuged (12,000 × g, 4°C, 10 min) and the resulting RNA pellet was washed with 75% (v v−1) ethanol (1.5 ml) and centrifuged again (7500 × g, 4°C, 5 min). Supernatants were discarded and the RNA pellets were air-dried (5–10 min), dissolved in diethyl pyrocarbonate (DEPC)-treated water (50–100 μl) and incubated (55–60°C) for 10 min. The concentration of isolated nuclei acids was determined spectrophotometrically by measuring the absorbance at 260 nm. All samples were thereafter stored at −80°C until required.
One-step RT–PCR method was employed in this study (QIAGEN® Onestep RT–PCR kit, Qiagen Ltd, Valencia, CA, U.S.A.). Total RNA template (1 μg) was mixed with 5 × RT–PCR buffer (4 μl), dNTP mix (400 μM, 0.8 μl), 1.2 μl of each primer (0.6 μM), enzyme mix (0.8 μl, a mixture of omniscript, sensiscript reverse transcriptases and HotStar Taq DNA polymerase) and DEPC-treated water. The final volume was 20 μl. For the detection of CSE mRNA, the forward primer sequence used was 5′-CATGGATGAAGTGTATGGAGGC-3′, and the reverse primer sequence was 5′-CGGCAGCAGAGGTAACAATCG-3′. For detection of CBS, the forward primer sequence used was 5′-GAGCGAGCCGGAACCTTGAAGC-3′, and the reverse primer sequence was 5′-CACCTATCCACCACCGCCCTGTC-3′. The PCR product size was 445 bp (CSE) and 579 bp (CBS). RT–PCR was performed at 50°C for 30 min and at 95°C for 15 min for reverse transcription followed by 29 cycles of PCR reaction consisting of 94°C (30 s) for denaturation, 58°C (30 s) for CSE primer-specific annealing and 60°C (30 s) for CBS primer-specific annealing, and 72°C (30 s) for extension. PCR products were analyzed by 15% (w v1) agarose gel electrophoresis and imaged by MultiGenius Bioimaging system (Syngene, Cambridge, U.K.). The band intensity was semiquantified by densitometry using gel analysis software (Syngene Ltd, Frederick, MD, U.S.A.) using glyceraldehyde-3-phosphate dehydrogenase as a marker gene.
Materials
PAG and E. coli LPS (serotype 0127:B8) were obtained from Sigma-Aldrich Company Ltd (Poole, Dorset, U.K.). Thiopentone sodium (Intraval Sodium®) was purchased from Rhône Mérieux Ltd (Harlow, Essex, U.K.). All stock solutions were prepared in nonpyrogenic saline (0.9% NaCl; Baxter Healthcare Ltd, Thetford, Norfolk, U.K.).
Statistical evaluation
All data are presented as means±s.e.m. of n observations, where n represents the number of animals or blood samples studied. For repeated measurements, a two-way analysis of variance (ANOVA) was performed. Data without repeated measurements was analyzed by one-way ANOVA, followed by a Dunnett's test for multiple comparisons. A P-value of less than 0.05 was considered to be statistically significant.
Results
Circulatory failure caused by endotoxemia
Baseline values of MAP and HR were similar in all of the animal groups studied and ranged from 122±6 to 128±5 mmHg (Figure 1a) and from 399±15 to 437±11 beats min−1 (Figure 1b), respectively. In animals without endotoxemia (sham-operated animals), injection of PAG did not result in any significant alterations in MAP or HR compared to Sham Control (Figure 1).
Figure 1.
Alterations in (a) mean arterial blood pressure (MAP) and (b) heart rate (HR) in rats subjected to the surgical procedure and pretreated with either saline (Sham Control, n=9) or DL-propargylglycine (Sham PAG, n=6). Rats subjected to endotoxemia (LPS 6 mg kg−1 i.v.) were pretreated with either saline (LPS Control, n=9) or PAG. PAG was administered either 30 min prior to the induction of endotoxemia at 10 mg kg−1 (LPS PAG 10 Pre, n=8) or 50 mg kg−1 (LPS PAG 50 Pre, n=9), or 60 min after the induction of endotoxemia at 50 mg kg−1 (LPS PAG 50 Post, n=9). *P<0.05 when compared with LPS Control.
Injection of LPS (6 mg kg−1 i.v. over 10 min) resulted in a modest biphasic fall in MAP from 125±6 mmHg (baseline) to 75±6 mmHg at 360 min (Figure 1a, P<0.05). Pretreatment of rats with PAG at the doses of 10–50 mg kg−1 did not affect the fall in MAP caused by endotoxemia (Figure 1a). An increase in HR was observed in rats subjected to endotoxemia and treated with vehicle (Figure 1b, P<0.05). The observed increase in HR was not affected by PAG at the doses of 10–50 mg kg−1 (Figure 1b). A higher dose of 100 mg kg−1 of PAG proved lethal (n=4, mortality rate 100% between 3 and 6 h).
Inhibition of H2S formation reduces organ injury caused by endotoxemia
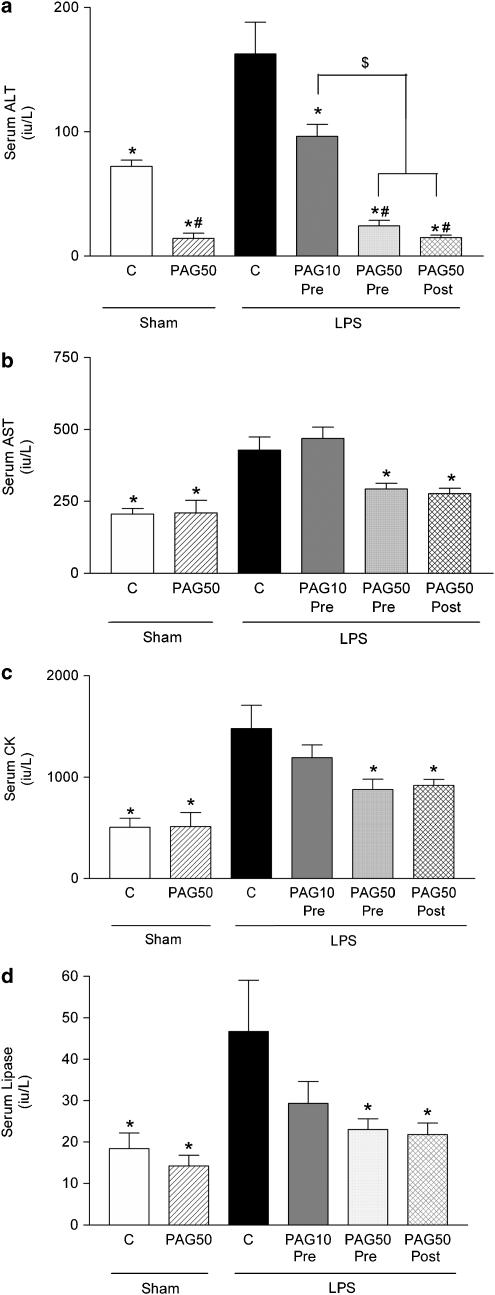
When compared to the rats treated with vehicle rather than LPS (Sham Control), endotoxemia resulted in significant rises in the serum levels of alanine transaminase (ALT) and aspartate transaminase (AST) (liver injury, Figure 2a and b, P<0.05), creatine kinase (CK) (neuromuscular injury, Figure 2c, P<0.05) and lipase (pancreatic injury, Figure 2d, P<0.05).
Figure 2.
Alterations in the serum levels of (a) alanine aminotransferase (ALT) and (b) aspartate aminotransferase (AST), (c) creatine kinase (CK) and (d) lipase in rats subjected to the surgical procedure and pretreated with either saline (Sham Control, n=9) or DL-propargylglycine (Sham PAG, n=6). Rats subjected to endotoxemia (LPS 6 mg kg−1 i.v.) were treated with either saline (LPS Control, n=9) or PAG. PAG was administered either 30 min prior to the induction of endotoxemia at 10 mg kg−1 (LPS PAG 10 Pre, n=8) or 50 mg kg−1 (LPS PAG 50 Pre, n=9), or 60 min after the induction of endotoxemia at 50 mg kg−1 (LPS PAG 50 Post, n=9). *P<0.05 when compared with LPS Control, #P<0.05 when compared with Sham Control, $P<0.05 when compared with LPS PAG 10 Pre.
Pre- or post-treatment with 50 mg kg−1 of PAG (LPS PAG 50 Pre or LPS PAG 50 Post, respectively) of rats subjected to endotoxemia attenuated the rises in the serum levels of ALT and AST and, hence, the liver injury caused by endotoxemia (Figure 2a and b, P<0.05). In addition, PAG at 50 mg kg−1 also attenuated the rises in the serum levels of CK (Figure 2c, P<0.05) and lipase (Figure 2d, P<0.05), and hence, respectively, the neuromuscular and pancreatic injury caused by endotoxemia. Pretreatment with a lower dose of PAG (10 mg kg−1) (LPS PAG 10 Pre) reduced the liver injury (measured as serum ALT levels). The serum levels of CK and lipase were also reduced by the lower dose of PAG, but these effects were not statistically significant. In sham-operated animals, administration of PAG at 50 mg kg−1 resulted in a reduction in the serum level of ALT when compared to sham-operated animals treated with saline (Figure 2a, P<0.05).
Endotoxemia increases MPO activity in the rat liver: effect of PAG
When compared to the sham-operated rats (Sham Control), endotoxemia (LPS Control) resulted in significant increases in the MPO activity in the liver, indicating increased leukocyte infiltration (Figure 3, P<0.05). Pretreatment of rats with PAG at 50 mg kg−1 (LPS PAG 50 Pre) significantly reduced the MPO activity in the liver.
Figure 3.
Myeloperoxidase (MPO) activity in the liver of rats subjected to the surgical procedure and pretreated with either saline (Sham Control) or DL-propargylglycine (Sham PAG). Rats subjected to endotoxemia (LPS 6 mg kg−1 i.v.) were pretreated with either saline (LPS Control) or PAG 30 min prior to (LPS PAG Pre) the induction of endotoxemia. N=6 for each group. *P<0.05 when compared with LPS Control.
Endotoxemia increases H2S-synthesizing activity in the rat liver: effect of PAG
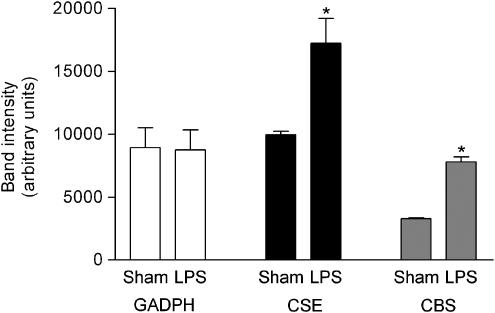
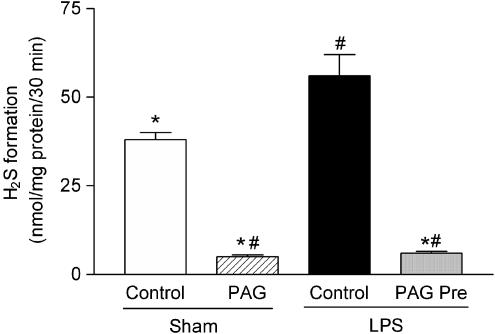
When compared to the sham-operated rats (Sham Control), endotoxemia (LPS Control) resulted in significant increases in the expression of H2S-forming enzymes CSE and CBS, as evidenced by RT–PCR of RNA extracted from the liver (Figure 4, P<0.05). In addition, incubation of rat liver homogenates with cysteine resulted in significantly increased formation of H2S in endotoxemic rats when compared to the sham-operated animals (Figure 5, P<0.05). Treatment of rats with PAG abolished the H2S synthesis in the rat liver.
Figure 4.
CSE and CBS mRNA expression in the liver of rats subjected to the surgical procedure only (Sham) or endotoxemia (LPS). N=6 for each group. *P<0.05 when compared with respective Sham.
Figure 5.
H2S formation in the liver of rats subjected to the surgical procedure and pretreated with either saline (Sham Control) or DL-propargylglycine (Sham PAG). Rats subjected to endotoxemia (LPS 6 mg kg−1 i.v.) were pretreated with either saline (LPS Control) or PAG 30 min prior to (LPS PAG Pre) the induction of endotoxemia. N=6 for each group. *P<0.05 when compared with LPS Control, #P<0.05 when compared with Sham Control.
Discussion
Severe sepsis and septic shock are important causes of death in intensive care units. The patient outcome correlates with the progression of shock to multiple organ failure and the number of organs failing (Baue, 1999). We demonstrate here that endotoxemia caused multiple organ injury, including a substantial increase in the serum concentrations of ALT and AST, indicating hepatocellular injury. Endotoxemia was also associated with an increase in the serum lipase, indicative of pancreatic injury, and an increase in serum CK, indicative of neuromuscular (brain tissue, skeletal or cardiac muscle tissue) injury. Here, we report that pretreatment with an inhibitor of H2S synthesis, PAG, attenuated the liver injury, neuromuscular injury and pancreatic injury caused by acute severe endotoxemia in the rat.
It has been suggested that many therapeutic approaches for septic shock have failed, as in experimental settings they have been given prophylactically rather than therapeutically. Therefore, we also investigated whether employing a post-treatment regimen, that is, administering PAG 60 min after the induction of endotoxemia, would afford protection against the associated organ injury. We found that the development of organ injury caused by acute severe endotoxemia was indeed attenuated by the therapeutic treatment with the inhibitor of H2S synthesis, PAG. This broader therapeutic window interestingly suggests a possibility for the use of H2S synthesis inhibitors in the treatment of systemic inflammatory response and its complications. Indeed, it was recently reported that therapeutic administration of PAG reduces the severity of cerulein-induced pancreatitis and affords protection against the associated lung injury in mice (Bhatia et al., 2005b).
As leukocyte recruitment plays a pivotal role in the pathogenesis of organ injury caused by LPS (Jaeschke et al., 1991; Tanaka et al., 1993; Klintman et al., 2002), we investigated whether the protection afforded by PAG against the organ injury and dysfunction caused by endotoxemia was associated with a reduction in the leukocyte recruitment. We report here that pretreatment with PAG of rats subjected to endotoxemia indeed reduced the MPO activity in the liver, indicating attenuated neutrophil infiltration in this organ. In accordance with our finding, it was reported (while this manuscript was under evaluation) that pretreatment with PAG of mice subjected to endotoxemia reduced the LPS-induced increase in the MPO activity and lung and liver tissue damage (Li et al., 2005).
H2S is a potent vasodilator in vitro and in vivo (Kimura, 2002; Wang, 2002). In the present study, however, inhibition of H2S synthesis by PAG at doses ranging from 10 to 50 mg kg−1 elicited no hemodynamic effects. This is in contrast with a previous study, which suggested that overproduction of H2S might play a role in the vascular consequences of sepsis or endotoxemia in the rat (Hui et al., 2003). In addition, another recent paper reported that inhibitors of H2S synthesis partially reduced the hypotensive response associated with severe hemorrhage (Mok et al., 2004). Nevertheless, as the circulatory failure induced by endotoxemia was not attenuated by PAG in the present study despite the dose-dependent reduction in organ injury and dysfunction, favorable hemodynamic effects are unlikely to have contributed to the protection of the organs afforded by the inhibition of H2S synthesis. However, the effects of H2S and inhibition of H2S synthesis in the microvasculature still warrant further investigation.
The protection against the organ injury afforded by PAG was most pronounced in the liver. Therefore, we investigated the expression and activity of the H2S-synthesizing enzymes CSE and CBS specifically in this organ. We found that the expression and the activity of these enzymes in the rat liver were significantly increased by endotoxemia. In agreement with this finding, increased H2S synthesis particularly in the liver has also been observed subsequent to severe hemorrhage in a rat model of hemorrhagic shock (Mok et al., 2004). The formation of H2S in the liver was completely abolished by the treatment of rats with PAG. As PAG elicits a complete and irreversible inactivation of CSE activity in vitro (Johnston et al., 1979), and a near complete inhibition of liver CSE enzyme activity in rodents (Uren et al., 1978), our finding supports the role of CSE as the enzyme mainly responsible for H2S formation outside the central nervous system, where, in turn, CBS is considered the main H2S-forming enzyme (Abe & Kimura, 1996; Chen et al., 1999; Zhao et al., 2003). Interestingly, our finding that inhibition of H2S synthesis lowered ALT (a specific marker for hepatic parenchymal injury) levels in sham-operated animals indicates basal H2S production in the liver. This finding is in accordance with a previous report, which suggested that the liver may be the major site in the body for H2S production, perhaps even responsible for maintaining H2S concentration in the circulation (Zhao et al., 2003).
In conclusion, our results support the view that H2S contributes to the organ injury caused by acute severe endotoxemia. It is likely that the H2S is formed by CSE, since treatment with PAG, an inhibitor of this enzyme, afforded protection against the multiple organ injury caused by LPS. We propose that inhibitors of H2S synthesis may be useful in the therapy of the organ injury associated with sepsis and shock.
Acknowledgments
This study was financially supported by the William Harvey Research Foundation, Medical Research Council, U.K., and Emil Aaltonen Foundation and Helsingin Sanomat Centennial Foundation, Finland. We thank the Agency for Science, Technology and Research (A*STAR) for the award of a Graduate Scholarship to FA and the Office of Life Science (OLS) of the National University of Singapore for financial support (Grant No. R-184-000-074-712).
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CBS
cystathionine-β-synthase
- CK
creatine kinase
- CO
carbon monoxide
- CSE
cystathionine-γ-lyase
- H2S
hydrogen sulfide
- HO-1
hemioxygenase
- HR
heart rate
- LPS
lipopolysaccharide
- MPO
myeloperoxidase
- NaHS
sodium hydrogen sulfide
- PAG
DL-propargylglycine
References
- ABE K., KIMURA H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUE A.E. Sepsis, multi-organ dysfunction syndrome (MODS) and multiple organ failure (MOF). Prevention is better than treatment. Minerva Anestesiol. 1999;65:477–480. [PubMed] [Google Scholar]
- BAUE A.E.The multiple organ or system failure syndrome Pathophysiology of Shock, Sepsis, and Organ Failure 1993Berlin: Springer Verlag; 1004–1008.ed. Schlag, G. & Redl, H. pp. [Google Scholar]
- BHATIA M., BRADY M., ZAGORSKI J., CHRISTMAS S.E., CAMPBELL F., NEOPTOLEMOS J.P., SLAVIN J. Treatment with neutralising antibody against cytokine induced neutrophil chemoattractant (CINC) protects rats against acute pancreatitis associated lung injury. Gut. 2000;47:838–844. doi: 10.1136/gut.47.6.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BHATIA M., SALUJA A.K., HOFBAUER B., FROSSARD J.L., LEE H.S., CASTAGLIUOLO I., WANG C.C., GERARD N., POTHOULAKIS C., STEER M.L. Role of substance P and the neurokinin 1 receptor in acute pancreatitis and pancreatitis-associated lung injury. Proc. Natl. Acad. Sci. U.S.A. 1998;95:4760–4765. doi: 10.1073/pnas.95.8.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BHATIA M., SIDHAPURIWALA J., MOOCHHALA S.M., MOORE P.K. Hydrogen sulphide is a mediator of carrageenan-induced hindpaw oedema in the rat. Br. J. Pharmacol. 2005a;145:141–144. doi: 10.1038/sj.bjp.0706186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BHATIA M., WONG F.L., FU D., LAU H.Y., MOOCHHALA S.M., MOORE P.K. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J. 2005b;19:623–625. doi: 10.1096/fj.04-3023fje. [DOI] [PubMed] [Google Scholar]
- CHEN P., PODDAR R., TIPA E.V., DIBELLO P.M., MORAVEC C.D., ROBINSON K., GREEN R., KRUGER W.D., GARROW T.A., JACOBSEN D.W. Homocysteine metabolism in cardiovascular cells and tissues: implications for hyperhomocysteinemia and cardiovascular disease. Adv. Enzyme Regul. 1999;39:93–109. doi: 10.1016/s0065-2571(98)00029-6. [DOI] [PubMed] [Google Scholar]
- CHENG Y., NDISANG J.F., TANG G., CAO K., WANG R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H2316–H2323. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- CHUNYU Z., JUNBAO D., DINGFANG B., HUI Y., XIUYING T., CHAOSHU T. The regulatory effect of hydrogen sulfide on hypoxic pulmonary hypertension in rats. Biochem. Biophys. Res. Commun. 2003;302:810–816. doi: 10.1016/s0006-291x(03)00256-0. [DOI] [PubMed] [Google Scholar]
- GENG B., YANG J., QI Y., ZHAO J., PANG Y., DU J., TANG C. H2S generated by heart in rat and its effects on cardiac function. Biochem. Biophys. Res. Commun. 2004;313:362–368. doi: 10.1016/j.bbrc.2003.11.130. [DOI] [PubMed] [Google Scholar]
- HEWETT J.A., JEAN P.A., KUNKEL S.L., ROTH R.A. Relationship between tumor necrosis factor-alpha and neutrophils in endotoxin-induced liver injury. Am. J. Physiol. 1993;265:G1011–G1015. doi: 10.1152/ajpgi.1993.265.6.G1011. [DOI] [PubMed] [Google Scholar]
- HOSOKI R., MATSUKI N., KIMURA H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem. Biophys. Res. Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- HUI Y., DU J., TANG C., BIN G., JIANG H. Changes in arterial hydrogen sulfide (H(2)S) content during septic shock and endotoxin shock in rats. J. Infect. 2003;47:155–160. doi: 10.1016/s0163-4453(03)00043-4. [DOI] [PubMed] [Google Scholar]
- JAESCHKE H., FARHOOD A., SMITH C.W. Neutrophil-induced liver cell injury in endotoxin shock is a CD11b/CD18-dependent mechanism. Am. J. Physiol. 1991;261:G1051–G1056. doi: 10.1152/ajpgi.1991.261.6.G1051. [DOI] [PubMed] [Google Scholar]
- JOHNSTON M., JANKOWSKI D., MARCOTTE P., TANAKA H., ESAKI N., SODA K., WALSH C. Suicide inactivation of bacterial cystathionine gamma-synthase and methionine gamma-lyase during processing of L-propargylglycine. Biochemistry. 1979;18:4690–4701. doi: 10.1021/bi00588a033. [DOI] [PubMed] [Google Scholar]
- KIMURA H. Hydrogen sulfide as a neuromodulator. Mol. Neurobiol. 2002;26:13–19. doi: 10.1385/MN:26:1:013. [DOI] [PubMed] [Google Scholar]
- KLINTMAN D., SCHRAMM R., MENGER M.D., THORLACIUS H. Leukocyte recruitment in hepatic injury: selectin-mediated leukocyte rolling is a prerequisite for CD18-dependent firm adhesion. J. Hepatol. 2002;36:53–59. doi: 10.1016/s0168-8278(01)00226-4. [DOI] [PubMed] [Google Scholar]
- LI L., BHATIA M., ZHU Y.Z., ZHU Y.C., RAMNATH R.D., WANG Z.J., ANUAR F.B., WHITEMAN M., SALTO-TELLEZ M., MOORE P.K. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J. 2005;19:1196–1198. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- MILLAR C.G., THIEMERMANN C. Carboxy-PTIO, a scavenger of nitric oxide, selectively inhibits the increase in medullary perfusion and improves renal function in endotoxemia. Shock. 2002;18:64–68. doi: 10.1097/00024382-200207000-00012. [DOI] [PubMed] [Google Scholar]
- MOK Y.Y., SHIRHAN BIN MOHAMMED A.M., YOKE P.C., ZHONG J.W., BHATIA M., MOOCHHALA S., MOORE P.K. Role of hydrogen sulphide in haemorrhagic shock in the rat: protective effect of inhibitors of hydrogen sulphide biosynthesis. Br. J. Pharmacol. 2004;143:881–889. doi: 10.1038/sj.bjp.0706014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE P.K., BHATIA M., MOOCHHALA S. Hydrogen sulfide: from the smell of the past to the mediator of the future. Trends Pharmacol. Sci. 2003;24:609–611. doi: 10.1016/j.tips.2003.10.007. [DOI] [PubMed] [Google Scholar]
- QINGYOU Z., JUNBAO D., WEIJIN Z., HUI Y., CHAOSHU T., CHUNYU Z. Impact of hydrogen sulfide on carbon monoxide/heme oxygenase pathway in the pathogenesis of hypoxic pulmonary hypertension. Biochem. Biophys. Res. Commun. 2004;317:30–37. doi: 10.1016/j.bbrc.2004.02.176. [DOI] [PubMed] [Google Scholar]
- RUETTEN H., SOUTHAN G.J., ABATE A., THIEMERMANN C. Attenuation of endotoxin-induced multiple organ dysfunction by 1-amino-2-hydroxy-guanidine, a potent inhibitor of inducible nitric oxide synthase. Br. J. Pharmacol. 1996;118:261–270. doi: 10.1111/j.1476-5381.1996.tb15397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUETTEN H., THIEMERMANN C. Effects of tyrphostins and genistein on the circulatory failure and organ dysfunction caused by endotoxin in the rat: a possible role for protein tyrosine kinase. Br. J. Pharmacol. 1997;122:59–70. doi: 10.1038/sj.bjp.0701345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STIPANUK M.H., BECK P.W. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem. J. 1982;206:267–277. doi: 10.1042/bj2060267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANAKA Y., KOBAYASHI K., TAKAHASHI A., ARAI I., HIGUCHI S., OTOMO S., HABU S., NISHIMURA T. Inhibition of inflammatory liver injury by a monoclonal antibody against lymphocyte function-associated antigen-1. J. Immunol. 1993;151:5088–5095. [PubMed] [Google Scholar]
- TEAGUE B., ASIEDU S., MOORE P.K. The smooth muscle relaxant effect of hydrogen sulphide in vitro: evidence for a physiological role to control intestinal contractility. Br. J Pharmacol. 2002;137:139–145. doi: 10.1038/sj.bjp.0704858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UREN J.R., RAGIN R., CHAYKOVSKY M. Modulation of cysteine metabolism in mice – effects of propargylglycine and L-cyst(e)ine-degrading enzymes. Biochem. Pharmacol. 1978;27:2807–2814. doi: 10.1016/0006-2952(78)90194-6. [DOI] [PubMed] [Google Scholar]
- WANG R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter. FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- ZHAO W., NDISANG J.F., WANG R. Modulation of endogenous production of H2S in rat tissues. Can. J. Physiol. Pharmacol. 2003;81:848–853. doi: 10.1139/y03-077. [DOI] [PubMed] [Google Scholar]
- ZHAO W., ZHANG J., LU Y., WANG R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]