Nonsteroidal anti-inflammatory drugs (NSAIDs) are currently the most widely used medications for the treatment of inflammatory diseases. NSAID development stemmed from the long-recognised analgesic properties associated with willow bark, ultimately leading to discovery of aspirin (acetyl salicylic acid), the best-known and most widely used NSAID. The primary mechanism of action of this class of drugs was first described by the Nobel Laureate, Sir John Vane in 1971 (Vane, 1971), and was shown to involve the inhibition of cyclooxygenase (COX), the enzyme responsible for production of prostaglandins from the precursor arachidonic acid. It has since been established that at least two isoforms of the enzyme exist: COX-1 is the widely expressed constitutive isoform responsible for the generation of prostaglandins involved in homeostasis and cell signalling while COX-2 is an inducible isoform found in activated inflammatory cells and generates prostanoid mediators of inflammation. It is widely accepted, therefore, that the anti-inflammatory effects of NSAIDs are through COX-2 inhibition, but that the gastrotoxicity, which limits their clinical use, is attributed to inhibition of COX-1. This feature has triggered the intense interest in development of COX-2 specific inhibitors, which were predicted to eliminate the COX-1-induced gastric complications, while retaining the desired anti-inflammatory effects. The relative merits of specific COX-2 inhibitors (e.g. celecoxib and rofecoxib) have, however, been limited with respect to their efficacy in the treatment of inflammatory diseases (Warner & Mitchell, 2004) and in cancer studies (Thun et al., 2002). Some researchers have found that the COX-2 specific compounds were not as effective, in terms of their anti-inflammatory properties, as the nonspecific COX inhibitors, such as indomethacin (Wallace, 1999). In addition, some of these COX-2 selective inhibitors have been withdrawn due to their adverse effects on the cardiovascular system (Bannwarth, 2005).
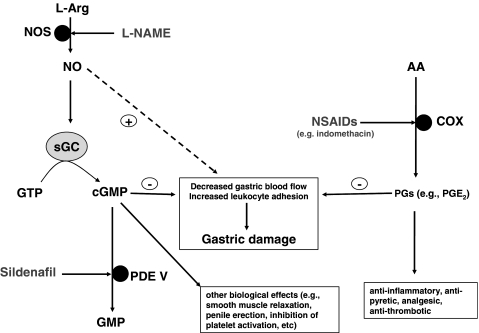
An alternative means of addressing the problem of NSAID-induced gastric injury is to ameliorate the adverse effects of reduced generation of protective prostaglandins (e.g. PGE2) via an alternative pathway. Nitric oxide (NO) shares many of the physiological properties of PGE2 in the gastric mucosa, particularly with respect to increased gastric blood flow and reduced inflammatory cell adhesion. These features of NO have been exploited in NO-adducts of NSAIDS (e.g. NO-aspirin), which have been shown to offer some protection against injury to the gastric mucosa by generating sufficient NO to substitute for deficient prostaglandins (Wallace et al., 2002). However, while low levels of NO offer protection against gastric injury via activation of soluble guanylate cyclase and generation of cyclic guanosine monophosphate (cGMP), high levels of NO have been shown to induce gastric injury (Muscara & Wallace, 1999). An interesting alternative approach to this problem is offered by Santos et al. (2005) in an elegant study published in this issue of the journal. This study shows that sildenafil (also known as Viagra™), an inhibitor of the enzyme responsible for breakdown of cGMP, phosphodiesterase V (PDE V), affords protection against indomethacin-induced gastropathy in rats. The fundamental findings of this study were that sildenafil dose-dependently reduced the gastric damage and augmented gastric neutrophil myeloperoxidase activity induced by indomethacin. Interestingly, the NOS inhibitor L-NAME dose-dependently reversed the protective effects of sildenafil. The effect of L-NAME was prevented when the NO precursor L-arginine was coadministered. Intravital microscopic assessment of indomethacin-induced increase in leucocyte adhesion in the mesentery venules was also decreased by sildenafil, a response inhibited by cotreatment of L-NAME. Importantly, sildenafil prevented indomethacin-induced blood flow without affecting the inhibitory effect of the NSAID on gastric PGE2 levels. The authors conclude that inhibition of PDE V by sildenafil leads to an increased prevalence of cGMP that has been generated in response to endogenous NO. Thus, increased levels of cGMP appear to compensate for the NSAID-mediated reduction in prostaglandin generation and protect against gastropathy (Figure 1).
Figure 1.
Nonsteroidal anti-inflammatory drugs (NSAIDs) such as aspirin and indomethacin inhibit cyclooxygenase (COX) enzymes to prevent the formation of prostaglandins (PGs) from the membrane lipid arachidonic acid (AA). It is primarily through inhibition of COX that NSAIDs are believed to exert their anti-inflammatory, antipyretic, analgesic and antithrombotic properties. Products of COX activity, especially PGE2, also act to limit gastric damage by increasing blood flow and reducing leukocyte adhesion. Inhibition of PG formation by NSAIDs therefore results in increased gastropathy. Sildenafil by inhibiting phosphodiesterase V (PDE V) prevents the breakdown of cyclic guanosine monophosphate (cGMP) to GMP. cGMP exerts may biological effects including smooth muscle relaxation, penile erection and inhibition of platelet activation. In addition, it also reduces gastric damage by augmenting gastric blood flow and limiting leukocyte adhesion. Nitric oxide (NO) formed by the action of NO synthase (NOS) enzymes on L-arginine (L-arg) acts upon soluble guanylate cyclase (sGC) to convert guanosine triphosphate (GTP) to cGMP. Thus, prevention of NO formation and therefore cGMP by NOS inhibitors such as L-NAME prevents the protective effects of sildenafil. Importantly, low levels of NO offer protection against gastric injury, but high levels can induce gastric injury.
This work suggests that coadministration of a PDE V inhibitor with an NSAID might offer a means of preventing the unwanted gastric side effects of NSAIDs. Perhaps, we should consider adding gastric protection to the lengthening list of therapeutic targets for sildenafil over and above its much-heralded primary target of impotence.
References
- BANNWARTH B. Do selective cyclo-oxygenase-2 inhibitors have a future. Drug Saf. 2005;28:183–189. doi: 10.2165/00002018-200528030-00001. [DOI] [PubMed] [Google Scholar]
- MUSCARA M.N., WALLACE J.L. Nitric oxide. V. therapeutic potential of nitric oxide donors and inhibitors. Am. J. Physiol. 1999;276:G1313–G1316. doi: 10.1152/ajpgi.1999.276.6.G1313. [DOI] [PubMed] [Google Scholar]
- SANTOS C.L., SOUZA M.H.L.P., GOMES A.S., LEMOS H.P., SANTOS A.A., CUNHA F.Q., WALLACE J.L.Sildenafil prevents indomethacin-induced gastropathy in rats: Role of leukocyte adherence and gastric blood flow Br. J. Pharmacol. 2005146481–486.in this issue [DOI] [PMC free article] [PubMed] [Google Scholar]
- THUN M.J., HENLEY S.J., PATRONO C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J. Natl. Cancer Inst. 2002;94:252–266. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- VANE J.R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat. New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L.Distribution and expression of cyclooxygenase (COX) isoenzymes, their physiological roles, and the categorization of nonsteroidal anti-inflammatory drugs (NSAIDs) Am. J. Med. 199910711S–16S.discussion 16S–17S [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., IGNARRO L.J., FIORUCCI S. Potential cardioprotective actions of no-releasing aspirin. Nat. Rev. Drug Discov. 2002;1:375–382. doi: 10.1038/nrd794. [DOI] [PubMed] [Google Scholar]
- WARNER T.D., MITCHELL J.A. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J. 2004;18:790–804. doi: 10.1096/fj.03-0645rev. [DOI] [PubMed] [Google Scholar]