Abstract
This study was undertaken to determine whether long-term in vivo administration of nitroglycerine (NTG) downregulates the hyperpolarization induced by acetylcholine (ACh) in aortic valve endothelial cells (AVECs) of the rabbit and, if so, whether antioxidant agents can normalize this downregulated hyperpolarization.
ACh (0.03–3 μM) induced a hyperpolarization through activations of both apamin- and charybdotoxin-sensitive Ca2+-activated K+ channels (KCa) in rabbit AVECs. The intermediate-conductance KCa channel (IKCa) activator 1-ethyl-2-benzimidazolinone (1-EBIO, 0.3 mM) induced a hyperpolarization of the same magnitude as ACh (3 μM).
The ACh-induced hyperpolarization was significantly weaker, although the ACh-induced [Ca2+]i increase was unchanged, in NTG-treated rabbits (versus NTG-untreated control rabbits). The hyperpolarization induced by 1-EBIO was also weaker in NTG-treated rabbits.
The reduced ACh-induced hyperpolarization seen in NTG-treated rabbits was not modified by in vitro application of the superoxide scavengers Mn-TBAP, tiron or ascorbate, but it was normalized when ascorbate was coadministered with NTG in vivo.
Superoxide production within the endothelial cell (estimated by ethidium fluorescence) was increased in NTG-treated rabbits and this increased production was normalized by in vivo coadministration of ascorbate with the NTG.
It is suggested that long-term in vivo administration of NTG downregulates the ACh-induced hyperpolarization in rabbit AVECs, possibly through chronic actions mediated by superoxide.
Keywords: Aortic valve, endothelial cell, nitrate tolerance, endothelium-dependent hyperpolarization, superoxide
Introduction
Vascular endothelial cells play an important role in the regulation of vascular tone through the release of vasorelaxing factors such as nitric oxide, prostacyclin and endothelium-derived hyperpolarizing factor (EDHF) (Kuriyama et al., 1998). In the endothelial cell, acetylcholine (ACh) activates Ca2+-activated K+ channels (KCa) through an increase in the intracellular concentration of Ca2+ ([Ca2+]i) (Himmel et al., 1994), thereby causing a membrane hyperpolarization (Brunet & Bény, 1989; Mehrke & Daut, 1990; Chen & Cheung, 1992; Marchenko & Sage, 1993; Carter & Ogden, 1994; Sharma & Davis, 1994; Frieden & Bény, 1995). Several types of KCa have been found to be expressed in endothelial cells (Chen & Cheung, 1992; Marchenko & Sage, 1996; Wang et al., 1996; Nilius et al., 1997). Among these, the intermediate-conductance KCa channel (IKCa) and/or small-conductance KCa channel (SKCa) have been suggested to be responsible for the ACh-induced hyperpolarization in vascular endothelial cells under physiological conditions (Busse et al., 2002; Bychkov et al., 2002; Eichler et al., 2003; Hinton & Langton, 2003).
Nitroglycerine (NTG) is widely used in the management of such cardiovascular diseases as angina pectoris, acute myocardial infarction and congestive heart failure. Despite their beneficial haemodynamic and anti-ischaemic effects, the usefulness of organic nitrates is limited by tolerance, which develops shortly after treatment starts (Parker et al., 1991). More importantly, long-term (e.g. 3–10 days) NTG therapy causes endothelial dysfunction in the coronary and forearm arterial beds in humans (Caramori et al., 1998; Gori et al., 2001). In rat and rabbit aortae, the relaxation responses to endothelium-derived nitric oxide decline after 3 days' in vivo administration of NTG (Münzel et al., 1995; Laursen et al., 1996; Berkenboom et al., 1999). In addition, the synthesis of prostacyclin is reduced in the aorta of rabbits treated in vivo with NTG (Hink et al., 2003). These observations suggest that both the nitric oxide and the prostacyclin derived from the endothelium undergo downregulations in function in arteries following chronic NTG administration. It has been suggested that an increased production of superoxide in vascular walls plays an important role in these dysfunctions affecting endothelium-derived relaxing factors in NTG-treated animals (Cai & Harrison, 2000; Münzel et al., 2000; Gori & Parker, 2002a, 2002b). However, it remains unclear whether long-term in vivo administration of NTG modulates the function of EDHF and, if so, whether superoxide plays a role in this modulation.
A hyperpolarization occurring in vascular endothelial cells is propagated directly to the underlying smooth muscle cells via myoendothelial coupling, the result being smooth muscle cell hyperpolarization (Griffith, 2004). This suggests that endothelial cell hyperpolarization is a pivotal event in the actions of EDHF. Conversely, membrane potential changes evoked in vascular smooth muscle cells can be propagated to endothelial cells via myoendothelial coupling (von der Weid & Bény, 1993; Bény & Pacicca, 1994; Marchenko & Sage, 1994; Bény, 1997; Griffith, 2004), indicating the difficulty of recording electrical activity that truly arises from endothelial cells when intact vascular tissues are used. We previously found that use of aortic valve endothelial cells (AVECs) obtained from rabbits allowed us to circumvent this problem (Ohashi et al., 1999). We therefore felt that rabbit AVECs might be a suitable preparation for an investigation of the effects of long-term in vivo NTG administration on the hyperpolarization of endothelial cells under ex vivo conditions.
In this study, we first examined whether the hyperpolarization induced in AVECs by ACh is reduced following long-term (10 days) in vivo NTG administration. We next examined whether in vivo NTG administration increases superoxide production by AVECs. The in vitro effects of the superoxide scavengers Mn-TBAP (Quijano et al., 2001), tiron and ascorbate on the ACh-induced hyperpolarization in such endothelial cells were also examined in NTG-treated rabbits. Finally, we examined the effect of in vivo coadministration of ascorbate with NTG on the ACh-induced hyperpolarization in AVECs.
Methods
Animals
All experiments performed in this study conformed to guidelines on the conduct of animal experiments issued by the Graduate School of Medical Sciences in Nagoya City University and were approved by the Committee on the Ethics of Animal Experiments in that institution. Male Japan White albino rabbits (supplied by Kitayama Labes, Ina, Japan), weighing 2.5–3.0 kg were treated by applying transdermal NTG patches (Nitroderm TTS; Novartis Pharma, Tokyo, Japan) to a shaved dorsal thoracic area of the body. Such patches were present continuously for a period of 10 days (each patch being replaced daily with a new one) (‘NTG-treated rabbits'). The theoretical delivery of NTG was 5 mg per 24 h. In some of these NTG-treated rabbits, ascorbate (1 g kg−1, Schwenke & Behr, 1998) suspended in 0.15% carboxymethylcellulose solution was administered orally once a day for the same 10-day period (‘NTG+ascorbate-treated rabbits'). Male rabbits of a similar body weight served as controls (‘control rabbits').
Tissue preparation
Rabbits were anaesthetized by injection of pentobarbitone sodium (40 mg kg−1 given i.v.), then killed by exsanguination. The heart was rapidly excised and placed in a chamber filled with Krebs' solution. The aorta was opened by a longitudinal incision at the attachment to the left ventricle and the aortic valve was dissected out under a binocular microscope.
Recording of membrane potential
The membrane potential of rabbit AVECs was measured using a conventional microelectrode technique, as reported previously (Ohashi et al., 1999). To allow recording of membrane potentials, each aortic valve was transferred to a chamber of 0.7 ml volume and superfused with warmed (37°C) Krebs' solution at a flow rate of 2 ml min−1. Glass microelectrodes were made from borosilicate glass tubing (OD=1.2 mm with a glass filament inside; Hilgenberg, Malsfeld, Germany) and filled with 1 M KCl. The resistance of the electrodes was 120–180 MΩ. The electrode was inserted into rabbit AVEC using a micromanipulator (model MHW-3; Narishige International, Tokyo, Japan). Membrane potentials, recorded using an Axoclamp-2B amplifier (Axon Instruments, Foster, CA, U.S.A.), were displayed on a cathode-ray oscilloscope (model VC-6020; Hitachi, Tokyo, Japan). Data were digitalized at an acquisition rate of 20 Hz using an AxoScope 9.0/Digidata 1200 data-acquisition system (Axon Instruments) and stored on an IBM/AT compatible PC.
To observe the concentration-dependent effects of ACh on the membrane potential, ACh (0.03–3 μM) was applied for 3 min at 25-min intervals in an ascending order in a given preparation. To examine the effect of KCa inhibitors on ACh-induced membrane potential changes, ACh (3 μM) was first applied for 3 min (to record the control response), followed by a 25-min washout. Iberiotoxin (IBTX, 0.1 μM), apamin (0.1 μM), charybdotoxin (CTX, 0.1 μM) or apamin (0.1 μM)+CTX (0.1 μM) was then applied for 5 min, and ACh was again applied in the presence of either agent. To examine the effects of a superoxide generator or one of several superoxide scavengers on ACh-induced membrane potential changes, the control ACh (3 μM)-response was first recorded, followed by a 25-min washout. This was followed by a 15-min application of (a) pyrogallol (100 μM, a superoxide generator) with or without the nitric oxide donor NOC-7 (10 μM) or (b) tiron (1 mM, a superoxide scavenger), Mn-TBAP (40 μM, a superoxide scavenger), ebselen (30 μM, a peroxynitrite scavenger), ascorbate (50 μM) or N-acetyl-L-cysteine (1 mM). Finally, ACh (3 μM) was again applied in the presence of the above agent(s). To examine the roles of endothelium-derived nitric oxide and prostacyclin on ACh-induced membrane potential changes, the control ACh (3 μM)-response was first recorded. The nitric-oxide-synthase inhibitor Nω-nitro-L-arginine (0.1 mM) or the cyclooxygenase inhibitor diclofenac (3 μM) was then applied for 30 min and ACh (3 μM) was again applied in the presence of either inhibitor.
Measurement of intracellular concentration of Ca2+ ([Ca2+]i)
The [Ca2+]i in rabbit AVECs was estimated using the ratiometric fluorescence Ca2+ indicator Fura-2. Fluorescence signals were detected using a CCD camera (C6790; Hamamatsu Photonics, Hamamatsu, Japan) fitted to an inverted fluorescence microscope (ECLIPSE TE300; Nikon, Tokyo, Japan). The microscope was equipped with a × 40 oil-immersion objective lens (NA 1.3; Nikon, Tokyo, Japan). First, AVECs were loaded with 5 μM Fura-2 acetoxymethyl ester (Fura-2 AM) in Krebs' solution containing 0.001% pluronic F-127 for 3 h at room temperature (20–23°C) under dark conditions. After loading, the aortic valve was placed in a chamber of 1 ml volume mounted on the fluorescence microscope and superfused with warmed (37°C) Krebs' solution at a flow rate of 4 ml min−1. The focus was adjusted to reveal individual AVECs and the experiment was started after 20–30 min superfusion. Various concentrations of ACh (0.03–3 μM) were then applied for 3 min with a 10-min interval in ascending order. Fura-2 was excited by dual wavelengths (340 nm (F340) and 380 nm (F380)) for 1 s and collected through a 510 nm emission filter (half-width, 20 nm) with a 15-s interval. The images were captured and analysed using commercial software (AquaCosmos; Hamamatsu Photonics). Changes in [Ca2+]i were expressed as the change in the fluorescence ratio F340/F380. The mean fluorescence intensities obtained from 10 AVECs in a given valve were averaged, and this (one value for each valve) was used for the later analysis.
Superoxide production
The oxidative fluorescence dye dihydroethidium (Molecular Probes, Eugene, OR, U.S.A.) was used to detect superoxide production. Dihydroethidium is oxidized by intracellular superoxide anion and converted to ethidium. This binds irreversibly to DNA, producing a bright red fluorescence. Segments of aortic valves were frozen in O.C.T. Compound (Tissue Tek; SAKURA Finetechnical, Tokyo, Japan) and kept at −80°C. Cryosections of the aortic valve were cut in the vertical plane at 10 μm thickness on a cryostat (Microtome Cryostat HM 550; MICROM International GmbH, Walldorf, Germany), then mounted on MAS-coated glass slides (Matsunami Glass, Kishiwada, Japan). The sections were then incubated in a light-protected chamber at 37°C for 30 min with 2 μM dihydroethidium in phosphate-buffered saline (PBS) solution (2.9 mM NaH2PO4, 9 mM Na2HPO4 and 137 mM NaCl, pH 7.2–7.4). The sections were viewed under an inverted microscope (Axiovert 100M; Carl Zeiss, Jena, Germany) equipped with a laser-scanning unit (LSM 5 PASCAL; Carl Zeiss). The images were controlled by a Pentium PC (32-bit Windows NT 4.0 operating system) running LSM 5 software (Carl Zeiss). The excitation wavelength was 488 nm and emission fluorescence was detected through a 585 nm long-pass filter. The fluorescence 12-bit images (512 × 512 pixels) from different groups of rabbits were acquired under identical conditions using LSM 5 software and then analysed with a digital image analyzer (Scion Image software, Scion Corp., Frederick, MD, U.S.A.). For each section of aortic valve, the total fluorescence intensity was calculated in four same-sized different areas (36 × 36 pixels) selected in a random manner and these being averaged. After background subtraction (using area containing no aortic valve section), relative fluorescence intensity was calculated using the aortic valve section obtained from ‘control rabbits' as standard.
CD31 staining
A monoclonal antibody against CD31 is quite specific for the staining of endothelial cells (Parums et al., 1990). Segments of aortic valves were fixed using 4% paraformaldehyde in PBS solution (pH 7.2–7.4) for 1 h. The segments were then embedded in O.C.T. Compound (Tissue Tek) and frozen at −80°C. Vertical sections of aortic valve (4 μm thickness) generated using a cryostat (Microtome Cryostat HM 550) were placed on MAS-coated glass slides (Matsunami Glass) for CD31 immuno-staining.
After a washout with PBS solution, the sections were incubated at 4°C overnight with monoclonal mouse anti-CD31 antibody (1 : 30 dilution; DakoCytomation, Glostrup, Denmark) as the primary antibody. After the sections had been rinsed with PBS, they were incubated with the secondary antibody (Alexa Fluor 568 goat anti-mouse IgG antibody; dilution 1 : 2000; Molecular Probes, Eugene, OR, U.S.A.) for 1 h at room temperature. After a further wash with PBS, the fluorescence of Alexa Fluor 568 was detected by confocal-laser-scanning system, as described above. The fluorescent 12-bit images from different groups of rabbits were acquired under identical conditions.
Solutions
The composition of the Krebs' solution was as follows (mM): 137.4 Na+, 5.9 K+, 1.2 Mg2+, 2.6 Ca2+, 15.5 HCO3−, 1.2 H2PO4−, 134 Cl−, 11.5 glucose. The solutions were bubbled with 95% oxygen and 5% carbon dioxide.
Drugs
The drugs used in the current experiments were as follows: ACh-hydrochloride (Daiichi Pharmaceutical, Tokyo, Japan), apamin, CTX, IBTX and Nω-nitro-L-arginine (Peptides Institute Inc., Osaka, Japan), NS-1619, ebselen, ionomycin, pyrogallol, and diclofenac sodium (Sigma Chemical Co., St Louis, MO, U.S.A.), 1-EBIO (Tocris Cookson, Ellisville, MO, U.S.A.), tiron, Fura-2 AM and NOC-7 (Dojindo Laboratories, Kumamoto, Japan), Mn-TBAP (Alexis Biochemicals, San Diego, CA, U.S.A.), L(+)-ascorbate (Wako, Osaka, Japan) and N-acetyl-L-cysteine (Nacalai Tesque, Kyoto, Japan).
Mn-TBAP was dissolved in ethanol (as a 50 mM stock solution). NOC-7 was dissolved in 0.1 N NaOH (as a 10 mM stock solution). 1-EBIO, Fura-2 AM, ionomycin and NS-1619 were dissolved in dimethylsulphoxide (DMSO) to concentrations of, respectively, 100, 1, 10 and 20 mM (each as a stock solution). The stock solutions were stored at −80°C and diluted in Krebs' solution to the required final concentrations immediately before use.
Control experiments indicated that none of the above solvents had any direct action at the final concentrations applied. All other stock solutions were made using ultrapure Milli-Q water (Japan Millipore Corp., Tokyo, Japan).
Statistical analysis
All results are expressed as the mean±s.e.m., with n values representing the number of rabbits used (each rabbit provided one segment for a given experiment). A one-way ANOVA (followed by Scheffé's F-test for post hoc analysis) or a Student's paired or unpaired t-test with an F-test were used for statistical analysis. The level of significance was set at P<0.05.
Results
ACh-induced membrane potential changes in AVECs from NTG-untreated control rabbits
ACh (3 μM) induced a transient followed by a sustained hyperpolarization, and a transient depolarization appeared after removal of ACh (Figure 1 and Table 1). These actions of ACh were concentration dependent (0.03–3 μM) (Figure 2 and Table 2).
Figure 1.
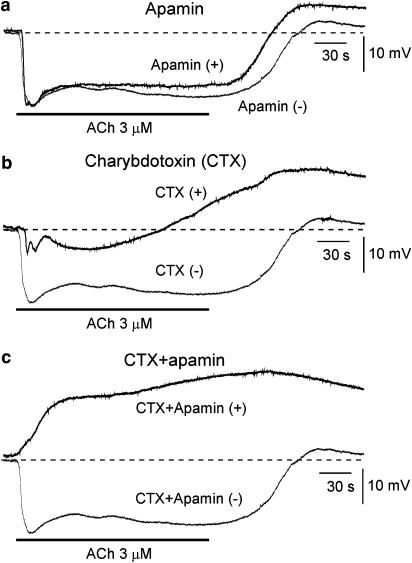
Effects of apamin, charybdotoxin (CTX) and apamin+CTX on acetylcholine (ACh)-induced membrane potential changes in aortic valve endothelial cells (AVECs) from NTG-untreated control rabbits. (a) Effects of apamin (0.1 μM). ACh (3 μM)-induced membrane potential changes before (‘Apamin (−)') and during (‘Apamin (+)') application of apamin. Resting membrane potentials (RMPs) were −51.5 mV and −51.0 mV before and during application of apamin, respectively. (b) Effects of CTX (0.1 μM). Before (‘CTX(−)') and during (‘CTX(+)') application of CTX. The RMPs were −51.5 and −50.6 mV before and during application of CTX, respectively. (c) Effects of CTX (0.1 μM)+apamin (0.1 μM). Before (‘CTX+Apamin (−)') and during (‘CTX+Apamin (+)') application of CTX+apamin. The RMPs were −51.5 and −50.0 mV before and during application of CTX+apamin, respectively. After recording the ACh-induced response, followed by a 25-min interval, one of the toxins or a combination was applied for 5 min, and ACh was then applied in the presence of the toxin(s). The two overlaying records shown in a given panel (a–c) were obtained from a single endothelial cell.
Table 1.
Effects of iberiotoxin (IBTX), apamin, charybdotoxin (CTX) and CTX+apamin on resting membrane potential (RMP) and acetylcholine (ACh)-induced membrane potential changes in aortic valve endothelial cells (AVECs) from NTG-untreated control rabbits
| n | RMP (mV) | Hyperpolarization (mV) | Depolarization (mV) | ||
|---|---|---|---|---|---|
| Transient | Sustained | ||||
| IBTX (0.1 μM) | 4 | ||||
| Before | −51.0±0.3 | 18.7±0.9 | 16.5±1.3 | 2.8±0.9 | |
| After | −50.7±0.4 | 19.0±0.8 | 16.6±0.7 | 3.2±0.8 | |
| Apamin (0.1 μM) | 5 | ||||
| Before | −50.8±0.4 | 19.3±1.0 | 17.4±0.8 | 3.2±0.7 | |
| After | −50.3±0.5 | 20.4±0.4 | 14.4±0.9* | 6.3±2.1 | |
| CTX (0.1 μM) | 4 | ||||
| Before | −51.4±0.3 | 19.2±0.6 | 16.8±0.7 | 2.2±1.2 | |
| After | −50.6±0.2 | 9.3±1.3** | 0.7±4.1** | 13.2±1.7** | |
| CTX (0.1 μM)+apamin (0.1 μM) | 4 | ||||
| Before | −51.2±0.3 | 18.0±0.7 | 15.8±1.2 | 3.3±1.0 | |
| After | −49.9±0.9 | 0** | 0** | 19.9±1.1** | |
Parentheses indicate concentration of toxin used. After the ACh (3 μM)-induced response had been recorded in the absence of toxin (before), one of the toxins or a combination of toxins was pretreated for 5 min. Finally, ACh was again applied in the presence of the toxin(s) (after). ACh was applied for 3 min with a 25-min interval to the same cells. The sustained hyperpolarization was measured 3 min after application of ACh, and the maximum depolarization was measured.
P<0.05
P<0.01 versus ‘before' in the same experiment (Student's paired t-test).
Figure 2.
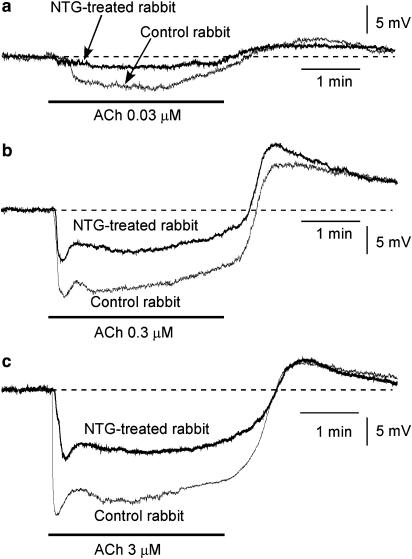
Effects of chronic in vivo administration of nitroglycerine (NTG) on ACh-induced membrane potential changes in AVECs. (a) Effects of 0.03 μM ACh on membrane potential in an AVEC from an NTG-untreated control rabbit (‘Control rabbit') or an NTG-treated rabbit (‘NTG-treated rabbit'). The RMP was −51.4 mV in Control rabbit and this being −51.5 mV in NTG-treated rabbit. (b) Effects of 0.3 μM ACh (as in a). The RMP was −51.2 mV in Control rabbit and this being −51.3 mV in NTG-treated rabbit. (c) Effects of 3 μM ACh (as in a). The RMP was −51.3 mV in Control rabbit and this being −51.4 mV in NTG-treated rabbit. The concentration-dependent effects of ACh shown in (a)–(c) were obtained from a single endothelial cell in each type of rabbit.
Table 2.
Effects of chronic administration of nitroglycerine (NTG), with or without ascorbate, on RMP and ACh-induced membrane potential changes in AVECs
| n | RMP (mV) | ACh (μM) | Hyperpolarization (mV) | Depolarization (mV) | ||
|---|---|---|---|---|---|---|
| Transient | Sustained | |||||
| NTG-untreated control rabbit | 10 | |||||
| −51.8±0.3 | 0.03 | 7.7±1.0 | 4.3±0.5 | 4.4±0.6 | ||
| −52.0±0.4 | 0.3 | 15.3±0.6 | 11.7±0.7 | 9.7±1.2 | ||
| −51.8±0.3 | 3 | 20.1±0.9 | 15.6±0.7 | 7.5±1.1 | ||
| NTG-treated rabbit | 10 | |||||
| −52.1±0.3 | 0.03 | 1.6±0.4** | 1.4±0.2* | 3.1±0.4 | ||
| −51.9±0.4 | 0.3 | 9.4±0.9** | 6.6±0.9** | 10.0±1.6 | ||
| −51.8±0.4 | 3 | 10.4±0.9** | 8.2±1.1** | 4.9±1.1 | ||
| NTG+ascorbate-treated rabbit | 4 | |||||
| −52.3±0.5 | 0.03 | 6.2±1.7 | 5.2±1.8 | 2.7±0.5 | ||
| −52.1±0.7 | 0.3 | 15.2±1.4 | 12.2±1.5 | 11.6±2.5 | ||
| −51.4±0.3 | 3 | 20.4±0.6 | 16.5±0.3 | 8.9±2.4 | ||
A given concentration of ACh (0.03–0.3 μM) was applied for 3 min with a 25-min interval. Ascorbate was administered in vivo with NTG for 10 days.
P<0.05
P<0.01 versus value at the corresponding ACh concentration in NTG-untreated control rabbits (one-way ANOVA and Scheffé's F-test).
The selective SKCa inhibitor apamin (0.1 μM) did not modify the resting membrane potential, although it significantly attenuated the ACh-induced sustained (but not transient) hyperpolarization (Figure 1a and Table 1). The selective inhibitor of the large-conductance Ca2+-activated K+ channel (BKCa) IBTX (0.1 μM) modified neither the resting membrane potential nor the ACh-induced transient or sustained hyperpolarization (Table 1). Charybdotoxin (CTX, 0.1 μM), a nonselective inhibitor of BKCa and IKCa, did not modify the resting membrane potential but it significantly attenuated both the transient and sustained hyperpolarizations induced by ACh (Figure 1b and Table 1). The membrane depolarization seen following ACh-removal was enhanced in the presence of CTX.
Coapplication of apamin (0.1 μM) and CTX (0.1 μM) did not significantly modify the resting membrane potential. In the presence of these two toxins, however, ACh induced a sustained depolarization (not a hyperpolarization) with a relatively slow onset (Figure 1c and Table 1).
The IKCa agonist 1-EBIO (0.3 mM; Edwards et al., 1999; Marrelli et al., 2003) induced a sustained hyperpolarization (18.2±0.8 mV, n=5) that was inhibited by CTX (1.3±0.5 mV, n=5; P<0.01) but not by IBTX (19.0±0.6 mV, n=5). The BKCa agonist NS-1619 (30 μM, Gauthier et al., 2002; Brakemeier et al., 2003) induced only a minimal hyperpolarization (1.8±0.3 mV, n=4).
The nitric-oxide-synthase inhibitor Nω-nitro-L-arginine modified neither the resting membrane potential (−51.7±0.2 mV to −51.9±0.4 mV, n=3; P>0.1) nor the ACh (3 μM)-induced hyperpolarization (for the transient response, 19.0±0.5 mV to 18.5±0.6 mV; for the sustained response, 15.2±0.4 mV to 15.3±0.6 mV, n=3; P>0.1 in each case). Similarly, the cyclooxygenase inhibitor diclofenac (3 μM) modified neither the resting membrane potential (−51.7±0.2 mV to −51.8±0.3 mV, n=3; P>0.1) nor the ACh (3 μM)-induced hyperpolarization (for the transient response, 19.0±0.5 mV to 18.7±0.4 mV; for the sustained response, 15.2±0.4 mV to 15.1±0.7 mV, n=3; P>0.1 in each case).
ACh-induced membrane potential changes in AVECs from NTG-treated rabbits
The resting membrane potential of AVECs from NTG-treated rabbits was not significantly different from that obtained for control rabbits (P>0.1, Table 2). In AVECs from NTG-treated rabbits, ACh (0.03–3 μM) concentration-dependently induced both a transient and a sustained hyperpolarization, with a transient depolarization appearing after ACh-removal, as also seen in AVECs from control rabbits (Figure 2). However, the transient and the sustained hyperpolarization were each significantly smaller in NTG-treated rabbits than in control rabbits, although the subsequent depolarization was not significantly different between the two groups (Figure 2 and Table 2).
Coapplication of CTX (0.1 μM) and apamin (0.1 μM) did not significantly modify the resting membrane potential (−51.4±0.4 mV to −50.7±0.8 mV, n=4; P>0.1) and in the presence of these two toxins, ACh produced a monotonic depolarization (17.0±0.9 mV, n=4).
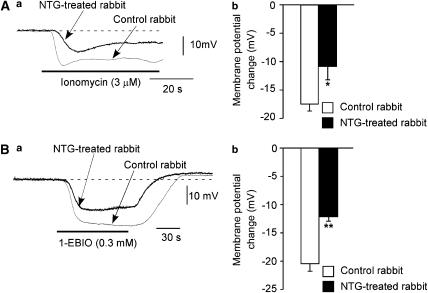
The Ca2+-ionophore ionomycin (3 μM) induced a hyperpolarization in AVECs from both groups of rabbits, although the hyperpolarization was significantly smaller in NTG-treated rabbits than in control rabbits (Figure 3A). Similarly, the hyperpolarization induced by the IKCa agonist 1-EBIO (0.3 mM) was significantly smaller in NTG-treated rabbits than in control rabbits (Figure 3B).
Figure 3.
Effects of chronic in vivo NTG administration on the hyperpolarization induced by the Ca2+-ionophore ionomycin or the IKCa agonist 1-EBIO in AVECs. (A) Effects of ionomycin (3 μM) on membrane potential in AVECs from NTG-untreated control rabbits (‘Control rabbit') and NTG-treated rabbits (‘NTG-treated rabbit'). (a) Actual traces. The RMP was −52.3 mV in Control rabbit and this being −52.2 mV in NTG-treated rabbit. (b) Summary of the effect of ionomycin on membrane potential. Mean of data from four different preparations (from four different animals) with s.e.m. (B) Effects of 1-EBIO (0.3 mM) (as in A). (a) Actual traces. The RMP was −52.5 mV in Control rabbit and this being −52.3 mV in NTG-treated rabbit. (b) Summary of the effect of 1-EBIO. Mean of data from six to seven different preparations (from six to seven different animals) with s.e.m. *P<0.05, **P<0.01 versus ‘Control rabbit'.
Effects of a superoxide generator and various superoxide scavengers on ACh-induced hyperpolarization
When the superoxide generator pyrogallol (0.1 mM, Marklund & Marklund, 1974; Indik et al., 2001) was applied in vitro for 15 min, it slightly depolarized the membrane and enhanced (not attenuated) both the transient and the sustained hyperpolarization induced by ACh (3 μM) in AVECs from control rabbits (Table 3). These actions of pyrogallol were enhanced when the nitric oxide donor NOC-7 (10 μM) was present (to generate peroxynitrite) (P<0.05 versus in the presence of pyrogallol alone).
Table 3.
Effects of a superoxide generator and several superoxide scavengers on ACh-induced hyperpolarization in AVECs
| n | RMP (mV) | Hyperpolarization (mV) | ||
|---|---|---|---|---|
| Transient | Sustained | |||
| NTG-untreated control rabbit | ||||
| Pyrogallol (0.1 mM) | 4 | |||
| Before | −51.6±0.2 | 18.4±0.4 | 15.3±0.4 | |
| After | −50.3±0.3* | 19.9±0.6* | 16.0±0.5* | |
| Pyrogallol (0.1 mM)+NOC-7 (10 μM) | 4 | |||
| Before | −51.9±0.7 | 18.2±0.7 | 15.9±1.0 | |
| After | −48.2±0.5*† | 21.6±0.3*† | 18.5±0.8*† | |
| NTG-treated rabbit | ||||
| Mn-TBAP (40 μM) | 4 | |||
| Before | −51.4±0.6 | 11.4±0.6 | 8.8±0.5 | |
| After | −52.7±0.5 | 10.6±0.5 | 7.4±0.8 | |
| Tiron (1 mM) | 4 | |||
| Before | −51.8±0.2 | 9.9±1.2 | 6.5±1.3 | |
| After | −54.2±0.7* | 8.4±0.5 | 3.4±1.6 | |
| Ebselen (30 μM) | 4 | |||
| Before | −52.4±0.4 | 10.3±0.9 | 6.7±1.5 | |
| After | −51.7±0.5 | 11.3±1.2 | 6.2±0.5 | |
| Ascorbate (50 μM, in vitro) | 3 | |||
| Before | −51.4±0.3 | 13.0±1.1 | 11.6±1.4 | |
| After | −51.5±0.5 | 12.5±1.2 | 10.2±1.5 | |
| N-acetyl-L-cysteine (1 mM) | 4 | |||
| Before | −52.3±0.5 | 10.8±0.8 | 7.6±1.7 | |
| After | −50.5±0.5 | 11.5±1.1 | 8.1±1.6 | |
After the ACh (3 μM)-induced hyperpolarization had been recorded in the absence of agent(s) (before), one agent or a combination of agents was pretreated for 15 min. Finally, ACh (3 μM) was again applied in the presence of the agent(s) (after). ACh was applied for 3 min with a 40-min interval to the same cells. Parentheses indicate the concentration of agent used.
P<0.05 versus ‘before' in the same experiment (Student's paired t-test).
P<0.05 versus in the presence of pyrogallol alone (Student's unpaired t-test).
In AVECs from control rabbits, in vitro application of the superoxide scavenger Mn-TBAP (40 μM) modified neither the resting membrane potential (−51.7±0.7 mV to −51.5±0.6 mV, n=3; P>0.1) nor the ACh-induced hyperpolarization (for the transient response, 19.2±0.5 mV to 18.9±0.7 mV; for the sustained response, 14.7±0.6 mV to 14.2±0.8 mV, n=3; P>0.1 in each case). Similarly, in AVECs from NTG-treated rabbits, in vitro application of Mn-TBAP (40 μM) modified neither the resting membrane potential nor the ACh (3 μM)-induced transient or sustained hyperpolarization (P>0.1) (Table 3). The other superoxide scavengers tested, tiron (1 mM) and ascorbate (50 μM), had no effect on either of the ACh-induced hyperpolarizations in AVECs from NTG-treated rabbits (although tiron itself slightly hyperpolarized the membrane, Table 3). In AVECs from NTG-treated rabbits, in vitro application of neither the peroxynitrite scavenger ebselen (30 μM; Daiber et al., 2000; Sawa et al., 2000) nor the intracellular sulphhydryl reducing agent N-acetyl-L-cysteine (1 mM; Zhang et al., 1994; Hashimoto et al., 2001) modified either the resting membrane potential or the ACh-induced hyperpolarizations (Table 3).
By contrast, when ascorbate was administered in vivo together with the NTG for 10 days, both the transient and the sustained hyperpolarization induced by ACh (0.03–3 μM) in AVECs were found to be normalized (Table 2).
ACh-induced increase in [Ca2+]i in AVECs
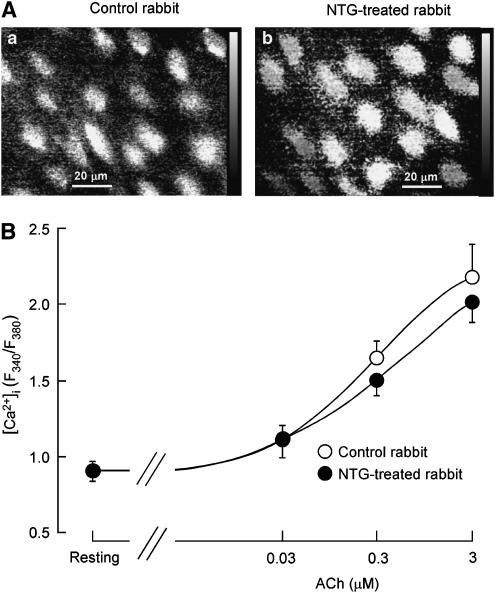
The resting value of the Fura-2 ratio was 0.91±0.07 (n=8) in AVECs from NTG-treated rabbits, a value similar to that obtained for those from control rabbits (0.91±0.06, n=7; P>0.5). ACh (0.03–3 μM) concentration-dependently increased [Ca2+]i in AVECs, the concentration–response curve being similar between NTG-treated rabbits and control rabbits (n=7–8; P>0.5, two-way repeated-measures ANOVA, Figure 4).
Figure 4.
Effects of in vivo chronic application of NTG on ACh-induced increase in intracellular concentration of Ca2+ ([Ca2+]i) in AVECs. (A) Increase in [Ca2+]i in AVECs at 3 min after application of ACh (3 μM). The [Ca2+]i is expressed as the Fura-2 ratio (F340/F380). (a) Control rabbit; (b) NTG-treated rabbit. (B) Concentration-dependent effects of ACh on [Ca2+]i in AVECs (from rabbits with or without NTG treatment). Each concentration of ACh was intermittently applied for 3 min with a 10-min interval. The maximum response induced by ACh at a given concentration was plotted. Mean of data from five different preparations (from five different animals) with s.e.m.
Superoxide production in aortic valves
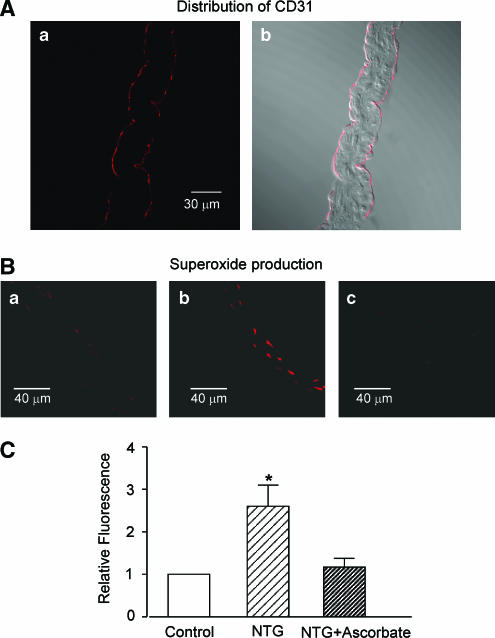
In cross-sections of the aortic valve, the fluorescence of the endothelial-cell marker CD31 (Krettek et al., 2004) was distributed close to the two sides of the preparation (Figure 5A). Superoxide production (estimated from the ethidium fluorescence intensity) was also detected in cells located near the edges of the preparation (Figure 5B). The ethidium fluorescence intensity was greatly increased in preparations from NTG-treated rabbits (Figure 5Bb) (as compared with that in the control group, Figure 5Ba), suggesting that superoxide production is increased in AVECs from NTG-treated rabbits (Figure 5C). This increased production of superoxide was normalized in rabbits in which ascorbate was coadministered with the NTG in vivo (Figure 5Bc and 5C).
Figure 5.
Superoxide production in aortic valves from rabbits treated in vivo, or not treated, with NTG or NTG+ascorbate. (A) Fluorescence image of antibody against the endothelial cell marker CD31(Aa) in a cross-section of an aortic valve, together with its bright field image (Ab). (B) Superoxide production in cross-sections of aortic valves. (Ba) NTG-untreated control rabbit; (Bb) NTG-treated rabbit; (Bc) NTG+ascorbate-treated rabbit. Superoxide was detected by dihydroethydium fluorescence. The images shown in (Ba)–(Bc) were taken under identical conditions. Similar results were obtained from four other preparations (from four different animals). (C) Summary of the effect of in vivo NTG administration on superoxide production. Relative fluorescence intensity was calculated using the aortic valve section from NTG-untreated control rabbit (Control) as standard. Mean of data from four different sections (from four different animals) with s.e.m. *P<0.05 versus ‘Control'.
Discussion
In the present experiments, on rabbit AVECs, ACh produced a transient, then a sustained hyperpolarization, with a subsequent transient depolarization after the removal of ACh. These results are consistent with previous findings in the endothelial cells of the rabbit aortic valve (Ohashi et al., 1999), rabbit aorta (Wang et al., 1996) and porcine coronary artery (Chen & Cheung, 1992). More importantly, we found that in vivo administration of NTG (for 10 days) significantly diminished both the transient and the sustained hyperpolarization without altering the depolarization seen after ACh-removal.
Characteristic features of ACh-induced hyperpolarization in AVECs
The ACh-induced hyperpolarization in rabbit AVECs is abolished in high K+ (50 mM) solution or upon application of the intracellular Ca2+-chelator acetoxymethyl ester of BAPTA (Ohashi et al., 1999). In the present experiments, we found that the Ca2+ ionophore ionomycin induced a hyperpolarization similar to that induced by 3 μM ACh in rabbit AVECs. These results suggest that in these AVECs, the ACh-induced hyperpolarization is due to activation of those K+ channels that are regulated by [Ca2+]i. Several types of Ca2+-activated K+ channels (KCa) have been identified in endothelial cells (Lückhoff & Busse, 1990; Sakai, 1990; Chen & Cheung, 1992; Sharma & Davis, 1994; Song & Davis, 1994; Nilius et al., 1997). In line with our previous findings (Ohashi et al., 1999), we found that in rabbit AVECs (a) the transient component of the ACh-induced hyperpolarization was markedly attenuated by CTX (a nonselective inhibitor of both BKCa and IKCa) but not by apamin (a selective inhibitor of SKCa) and (b) the sustained component of the ACh-induced hyperpolarization was inhibited by CTX and also by apamin. By contrast, the selective BKCa inhibitor IBTX modified neither the transient nor the sustained hyperpolarization. In addition, the IKCa agonist l-EBIO, but not the BKCa agonist NS-1619, induced a sustained hyperpolarization in rabbit AVECs. Moreover, coapplication of CTX and apamin completely blocked both components of the ACh-induced hyperpolarization in the present cells. These results suggest that in rabbit AVECs, the ACh-induced transient hyperpolarization may be due to an activation of IKCa, whereas the sustained hyperpolarization is due to activation of both IKCa and SKCa.
In the present experiments, neither the nitric-oxide-synthase inhibitor Nω-nitro-L-arginine nor the cyclooxygenase inhibitor diclofenac modified the ACh-induced hyperpolarization in rabbit AVECs. The results suggest that nitric oxide or prostacyclin derived from endothelial cells does not contribute to the ACh-induced hyperpolarization in rabbit AVECs.
Reduction of ACh-induced hyperpolarization in AVECs by in vivo NTG administration
Endothelial cells release vasorelaxing factors such as nitric oxide, prostacyclin and EDHF, and thereby regulate vascular tone (Kuriyama et al., 1998). It has been found that NTG administration in vivo leads to reductions in the relaxations to nitrovasodilators, as well as to endothelium-derived nitric oxide (referred to hereafter as ‘cross-tolerance'; Münzel et al., 1995; Gori & Parker, 2002a, 2002b). Furthermore, it was recently found that in the setting of ‘cross-tolerance', the synthesis of prostacyclin in the rabbit aorta is also downregulated (via an inhibition of its synthase by peroxynitrite (which is formed by the interaction of superoxide with nitric oxide)) (Hink et al., 2003). In the present experiments on rabbit AVECs, we found that (i) chronic in vivo NTG administration downregulated both the transient and the sustained hyperpolarization induced by ACh under conditions in which the endothelial [Ca2+]i level did not change, (ii) the muscarinic-receptor-independent hyperpolarization induced by either the Ca2+-ionophore ionomycin or the IKCa agonist 1-EBIO was also downregulated by chronic in vivo NTG administration. In a study employing the same protocol for chronic NTG administration, we previously found that the relaxation response to the nitric oxide donor NOC-7 in rabbit mesenteric resistance arteries (Nakano et al., 2004) and that to endothelium-derived nitric oxide in intrapulmonary veins (Kusama et al., 2005) are each downregulated. It is suggested that vascular endothelial hyperpolarization is directly propagated to the underlying smooth muscle cells via myoendothelial coupling (Griffith et al., 2004). Furthermore, it was found that the ACh-evoked smooth muscle cell hyperpolarizations in rabbit iliac artery correspond closely in magnitude and time course to those in rabbit AVECs (Ohashi et al., 1999; Griffith et al., 2002), suggesting that the membrane potentials in AVECs recorded in the present experiments may be reminiscent of many other endothelial cells. Taken together, the above results suggest that in addition to dysfunctions of nitric oxide and prostacyclin, the function of EDHF could also be downregulated in some types of vascular beds in the setting of ‘cross-tolerance'. Furthermore, the present results suggest that in rabbit AVECs, the observed downregulation of ACh-induced endothelial hyperpolarization may result from intervention at a step(s) between the induced [Ca2+]i increase and the subsequent activation of IKCa (and possibly SKCa). However, it should be noted that AVECs may not always behave as a typical vascular endothelial cells because of lacking smooth muscle cell contact.
It has been found that in the setting of ‘cross-tolerance', superoxide production via activation of membrane-bound NAD(P)H oxidase is increased in both vascular endothelial and smooth muscle cells (Münzel et al., 1995; Gori & Parker, 2002a, 2002b), suggesting a causal relationship between the increased superoxide production and the development of endothelial dysfunction in nitrate tolerance (Münzel et al., 1995; Gori & Parker, 2002a, 2002b). In vascular endothelial and smooth muscle cells, reactive oxygen species have been proposed to be regulators of the functions of various types of K+ channels, such as the ATP-sensitive (KATP), inward-rectifying (Kir) and Ca2+-activated (KCa) K+ channels (Cai & Sauvé, 1997). For example, superoxide inhibits the vasodilatations induced by the KATP agonist cromakalim and by the KCa agonist NS-1619 in the pial artery of newborn pigs (Ross & Armstead, 2003). Furthermore, reactive oxygen species (in particular H2O2) irreversibly inactivate BKCa in porcine renal artery endothelial cells (Brakemeier et al., 2003) as well as IKCa in bovine aorta endothelial cells (Cai & Sauvé, 1997). By contrast, in human umbilical vein endothelial cells, superoxide activates BKCa while low concentrations (0.01–0.25 μM) of H2O2 inhibit Kir (Bychkov et al., 1999). Moreover, peroxynitrite, but not superoxide, directly inhibits BKCa activity in the smooth muscle cells of both human coronary arterioles (Liu et al., 2002) and rat cerebral artery (Brzezinska et al., 2000). Thus, inconsistent (i.e. inhibitory as well as stimulatory) effects of reactive oxygen species on the function of KCa have been found in vascular cells (Cai & Sauvé, 1997; Bychkov et al., 1999).
In the present experiments, we found that chronic in vivo NTG administration greatly enhanced superoxide production (as assessed using dihydroethidium fluorescence) in rabbit AVECs. However, application of the superoxide generator pyrogallol in vitro, whether in the presence or absence of the nitric oxide donor NOC-7, resulted in a minor depolarization but no inhibition of the hyperpolarization induced by ACh in AVECs from control rabbits. Moreover, neither the IKCa inhibitor CTX nor the SKCa inhibitor apamin modified the resting membrane potential in rabbit AVECs. These results suggest that in rabbit AVECs, an acute increase in superoxide or peroxynitrite inhibits some of the K+ channels that regulate the resting membrane potential but has no significant effect on IKCa (or possibly SKCa) activity. Furthermore, we also found that the reduced ACh-induced hyperpolarization seen in AVECs from rabbits treated in vivo with NTG was not modulated by in vitro application of either an antioxidative agent (namely, Mn-TBAP, tiron or ascorbate) or the peroxinitrite scavenger ebselen. It is known that the oxidation state of sulphhydryl groups constitutes a determinant factor in K+-channel activity. For example, in bovine aortic endothelial cells, application of a hydrophilic oxidative agent such as 5,5′-dithio-bis(2-nitrobenzoic acid) or [(O-carboxyphenyl)thio]ethyl mercury sodium salt irreversibly reduces IKCa activity, an inhibitory effect that can be partly blocked by application of either the sulphhydryl-reducing agent dithiothreitol or reduced glutathione (Cai & Sauvé, 1997). In the present experiments, however, the intracellular sulphhydryl reducing agent N-acetyl-L-cysteine did not modify the reduced ACh-induced hyperpolarization seen in AVECs obtained from rabbits treated in vivo with NTG. Surprisingly, we found that coadministration of ascorbate with the NTG in vivo normalized this reduced ACh-induced hyperpolarization. Although in the present experiments we did not examine the effect of in vivo administration of ascorbate on the ACh-induced hyperpolarization in NTG-untreated control rabbits, it was found that this treatment does not affect the ACh-induced endothelium-dependent relaxation in normal rabbits (Mays et al., 1999). These results suggest that chronic effects of superoxide may be required to downregulate IKCa (and possibly SKCa) activities in rabbit AVECs. Such a downregulation could occur via a decrease in the total/functional number of IKCa or via a modulation of critical sulphhydryl groups either in the IKCa themselves and/or in functional proteins involved in the regulation of IKCa. However, clarification of the underlying mechanisms must await future work.
It has been found that ACh induces not only hyperpolarization but also depolarization in endothelial cells (Marchenko & Sage, 1993; Ohashi et al., 1999). The latter effect is thought to be due to activation of both Cl− transporters (Klein & O'Neill, 1990; Perry & O'Neill, 1993) and Cl− channels (Nilius et al., 1997). In the present experiments, we found that in rabbit AVECs, a transient depolarization occurred after removal of ACh and that in the combined presence of CTX and apamin, ACh evoked only a sustained depolarization. These results suggest that the depolarization phase is being generated while ACh is still present but that it is normally masked by the hyperpolarization. It is thought that modulation of Cl−-channel activity may be important in regulating the membrane potential in endothelial cells (Nilius et al., 1997). On this basis, a reduction in ACh-induced hyperpolarization in endothelial cells in NTG-treated rabbits could conceivably be due to an enhancement of Cl−-channel activity. However, this is unlikely to be true in rabbit AVECs because (i) the resting membrane potential in AVECs did not differ significantly between NTG-treated rabbits and control rabbits and (ii) the ACh-induced depolarization in the combined presence of CTX and apamin did not differ significantly between the two groups of rabbits. In the present experiments, the transient depolarization observed after removal of ACh did not differ significantly between the two groups. We found that in AVECs from control rabbits CTX blocked the ACh-induced sustained hyperpolarization and enhanced the ACh-induced depolarization. While, apamin significantly attenuated the ACh-induced sustained hyperpolarization but had no effect on the ACh-induced depolarization. Thus, it is suggested that the inhibition of ACh-induced hyperpolarization in NTG-treated rabbits may not be enough to enhance the ACh-induced depolarization.
In conclusion, in rabbit AVECs application of ACh produces a transient followed by a sustained hyperpolarization due to coactivation of CTX-sensitive and apamin-sensitive KCa. Both of the ACh-induced hyperpolarizations are downregulated in rabbits treated for 10 days in vivo with NTG. These downregulated hyperpolarizations are normalized by in vivo coadministration of the antioxidant ascorbate with the NTG but not by in vitro application of this antioxidant. Thus, we suggest that superoxide plays a pathophysiological role in the development and/or maintenance of the reduced membrane hyperpolarizations seen in AVECs obtained from nitrate-tolerant rabbits.
Acknowledgments
We thank Dr R.J. Timms for a critical reading of the manuscript and Professor T. Tada for advice on the immuno-histochemical experiments. This work was partly supported by a Grant-In-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan.
Abbreviations
- ACh
acetylcholine
- AVEC
aortic valve endothelial cell
- BKCa
large-conductance Ca2+-activated K+ channel
- CTX
charybdotoxin
- 1-EBIO
1-ethyl-2-benzimidazolinone
- EDHF
endothelium-derived hyperpolarizing factor
- Fura-2 AM
Fura-2 acetoxymethyl ester
- IBTX
iberiotoxin
- IKCa
intermediate-conductance Ca2+-activated K+ channel
- KATP
ATP-sensitive K+ channel
- KCa
Ca2+-activated K+ channel
- Kir
inward-rectifying K+ channels
- Mn-TBAP
manganese (III) tetrakis-(4-benzoic acid) porphyrin
- NOC-7
1-hydroxy-2-oxo-3-(N-methyl-3-aminopropyl)-3-methyl-1-triazene
- NS-1619
1,3-dihydro-1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-[trifluoromethyl]-2H-benzimidazol-2-one
- NTG
nitroglycerine
- SKCa
small-conductance Ca2+-activated K+ channel
- tiron
4,5-dihydroxy-1,3-benzene-disulphonic acid
References
- BÉNY J. Electrical coupling between smooth muscle cells and endothelial cells in pig coronary arteries. Pflügers Arch. 1997;433:364–367. doi: 10.1007/s004240050289. [DOI] [PubMed] [Google Scholar]
- BÉNY J., PACICCA C. Bidirectional electrical communication between smooth muscle and endothelial cells in the pig coronary artery. Am. J. Physiol. 1994;266:H1465–H1472. doi: 10.1152/ajpheart.1994.266.4.H1465. [DOI] [PubMed] [Google Scholar]
- BERKENBOOM G., FONTAINE D., UNGER P., BALDASSARRE S., PREUMONT N., FONTAINE J. Absence of nitrate tolerance after long-term treatment with ramipril: an endothelium-dependent mechanism. J. Cardiovasc. Pharmacol. 1999;34:547–553. doi: 10.1097/00005344-199910000-00011. [DOI] [PubMed] [Google Scholar]
- BRAKEMEIER S., EICHLER I., KNORR A., FASSHEBER T., KOHLER R., HOYER J. Modulation of Ca2+-activated K+ channel in renal artery endothelium in situ by nitric oxide and reactive oxygen species. Kidney Int. 2003;64:199–207. doi: 10.1046/j.1523-1755.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- BRUNET P., BÉNY J. Substance P and bradykinin hyperpolarize pig coronary artery endothelial cells in primary culture. Blood Vessels. 1989;26:228–234. doi: 10.1159/000158770. [DOI] [PubMed] [Google Scholar]
- BRZEZINSKA A., GEBREMEDHIN D., CHILIAN W., KALYANARAMAN B., ELLIOTT S. Peroxynitrite reversibly inhibits Ca2+-activated K+ channels in rat cerebral artery smooth muscle cells. Am. J. Physiol. 2000;278:H1883–H1890. doi: 10.1152/ajpheart.2000.278.6.H1883. [DOI] [PubMed] [Google Scholar]
- BUSSE R., EDWARDS G., FELETOU M., FLEMING I., VANHOUTTE P., WESTON A. EDHF: bringing the concepts together. Trends Pharmacol. Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- BYCHKOV R., BURNHAM M., RICHARDS G., EDWARDS G., WESTON A., FELETOU M., VANHOUTTE P. Characterization of a charybdotoxin-sensitive intermediate conductance Ca2+-activated K+ channel in porcine coronary endothelium: relevance to EDHF. Br. J. Pharmacol. 2002;137:1346–1354. doi: 10.1038/sj.bjp.0705057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BYCHKOV R., PIEPER K., RIED C., MILOSHEVA M., BYCHKOV E., LUFT F., HALLER H. Hydrogen peroxide, potassium currents, and membrane potential in human endothelial cells. Circulation. 1999;99:1719–1725. doi: 10.1161/01.cir.99.13.1719. [DOI] [PubMed] [Google Scholar]
- CAI H., HARRISON D. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ. Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- CAI S., SAUVÉ R. Effects of thiol-modifying agents on a K(Ca2+) channel of intermediate conductance in bovine aortic endothelial cells. J. Membr. Biol. 1997;158:147–158. doi: 10.1007/s002329900252. [DOI] [PubMed] [Google Scholar]
- CARAMORI P., ADELMAN A., AZEVEDO E., NEWTON G., PARKER A., PARKER J. Therapy with nitroglycerin increases coronary vasoconstriction in response to acetylcholine. J. Am. Coll. Cardiol. 1998;32:1969–1974. doi: 10.1016/s0735-1097(98)00456-2. [DOI] [PubMed] [Google Scholar]
- CARTER T., OGDEN D. Acetylcholine-stimulated changes of membrane potential and intracellular Ca2+ concentration recorded in endothelial cells in situ in the isolated rat aorta. Pflügers Arch. 1994;428:476–484. doi: 10.1007/BF00374568. [DOI] [PubMed] [Google Scholar]
- CHEN G., CHEUNG D. Characterization of acetylcholine-induced membrane hyperpolarization in endothelial cells. Circ. Res. 1992;70:257–263. doi: 10.1161/01.res.70.2.257. [DOI] [PubMed] [Google Scholar]
- DAIBER A., ZOU M., BACHSCHMID M., ULLRICH V. Ebselen as a peroxynitrite scavenger in vitro and ex vivo. Biochem. Pharmacol. 2000;59:153–160. doi: 10.1016/s0006-2952(99)00309-3. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., GARDENER M., FELETOU M., BRADY G., VANHOUTTE P., WESTON A. Further investigation of endothelium-derived hyperpolarizing factor (EDHF) in rat hepatic artery: studies using 1-EBIO and ouabain. Br. J. Pharmacol. 1999;128:1064–1070. doi: 10.1038/sj.bjp.0702916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EICHLER I., WIBAWA J., GRGIC I., KNORR A., BRAKEMEIER S., PRIES A., HOYER J., KOHLER R. Selective blockade of endothelial Ca2+-activated small- and intermediate-conductance K+-channels suppresses EDHF-mediated vasodilation. Br. J. Pharmacol. 2003;138:594–601. doi: 10.1038/sj.bjp.0705075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDEN M., BÉNY J. Effect of 5-hydroxytryptamine on the membrane potential of endothelial and smooth muscle cells in the pig coronary artery. Br. J. Pharmacol. 1995;115:95–100. doi: 10.1111/j.1476-5381.1995.tb16325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAUTHIER K., DEETER C., KRISHNA U., REDDY Y., BONDLELA M., FALCK J., CAMPBELL W. 14,15-Epoxyeicosa-5(Z)-enoic acid: a selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ. Res. 2002;90:1028–1036. doi: 10.1161/01.res.0000018162.87285.f8. [DOI] [PubMed] [Google Scholar]
- GORI T., MAK S., KELLY S., PARKER J. Evidence supporting abnormalities in nitric oxide synthase function induced by nitroglycerin in humans. J. Am. Coll. Cardiol. 2001;38:1096–1101. doi: 10.1016/s0735-1097(01)01510-8. [DOI] [PubMed] [Google Scholar]
- GORI T., PARKER J. Nitrate tolerance: a unifying hypothesis. Circulation. 2002a;106:2510–2513. doi: 10.1161/01.cir.0000036743.07406.53. [DOI] [PubMed] [Google Scholar]
- GORI T., PARKER J. The puzzle of nitrate tolerance: pieces smaller than we thought. Circulation. 2002b;106:2404–2408. doi: 10.1161/01.cir.0000036742.52907.91. [DOI] [PubMed] [Google Scholar]
- GRIFFITH T.M. Endothelium-dependent smooth muscle hyperpolarization: do gap junctions provide a unifying hypothesis. Br. J. Pharmacol. 2004;141:881–903. doi: 10.1038/sj.bjp.0705698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIFFITH T.M., CHAYTOR A.T., EDWARDS D.H. The obligatory link: role of gap junctional communication in endothelium-dependent smooth muscle hyperpolarization. Pharmacol. Res. 2004;49:551–564. doi: 10.1016/j.phrs.2003.11.014. [DOI] [PubMed] [Google Scholar]
- GRIFFITH T.M., CHAYTOR A.T., TAYLOR H.J., GIDDINGS B.D., EDWARDS D.H. cAMP facilitates EDHF-type relaxations in conduit arteries by enhancing electronic conduction via gap junctions. Proc. Natl. Acad. Sci. U.S.A. 2002;99:6392–6397. doi: 10.1073/pnas.092089799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHIMOTO S., GON Y., MATSUMOTO K., TAKESHITA I., HORIE T. N-acetylcysteine attenuates TNF-alpha-induced p38 MAP kinase activation and p38 MAP kinase-mediated IL-8 production by human pulmonary vascular endothelial cells. Br. J. Pharmacol. 2001;132:270–276. doi: 10.1038/sj.bjp.0703787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIMMEL H., RASMUSSON R., STRAUSS H. Agonist-induced changes of [Ca2+]i and membrane currents in single bovine aortic endothelial cells. Am. J. Physiol. 1994;267:C1338–C1350. doi: 10.1152/ajpcell.1994.267.5.C1338. [DOI] [PubMed] [Google Scholar]
- HINK U., OELZE M., KOLB P., BACHSCHMID M., ZOU M., DAIBER A., MOLLNAU H., AUGUST M., BALDUS S., TSILIMINGAS N., WALTER U., ULLRICH V., MÜNZEL T. Role for peroxynitrite in the inhibition of prostacyclin synthase in nitrate tolerance. J. Am. Coll. Cardiol. 2003;42:1826–1834. doi: 10.1016/j.jacc.2003.07.009. [DOI] [PubMed] [Google Scholar]
- HINTON J., LANGTON P. Inhibition of EDHF by two new combinations of K+-channel inhibitors in rat isolated mesenteric arteries. Br. J. Pharmacol. 2003;138:1031–1035. doi: 10.1038/sj.bjp.0705171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INDIK J., GOLDMAN S., GABALLA M. Oxidative stress contributes to vascular endothelial dysfunction in heart failure. Am. J. Physiol. 2001;281:H1767–H1770. doi: 10.1152/ajpheart.2001.281.4.H1767. [DOI] [PubMed] [Google Scholar]
- KLEIN J., O'NEILL W. Effect of bradykinin on Na-K-2Cl cotransport and bumetanide binding in aortic endothelial cells. J. Biol. Chem. 1990;265:22238–22242. [PubMed] [Google Scholar]
- KRETTEK A., SUKHOVA G., SCHONBECK U., LIBBY P. Enhanced expression of CD44 variants in human atheroma and abdominal aortic aneurysm: possible role for a feedback loop in endothelial cells. Am. J. Pathol. 2004;165:1571–1581. doi: 10.1016/S0002-9440(10)63414-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURIYAMA H., KITAMURA K., ITOH T., INOUE R. Physiological features of visceral smooth muscle cells, with special reference to receptors and ion channels. Physiol. Rev. 1998;78:811–920. doi: 10.1152/physrev.1998.78.3.811. [DOI] [PubMed] [Google Scholar]
- KUSAMA N., KAJIKURI J., WATANABE Y., SUZUKI Y., KATSUYA H., ITOH T. Characteristics of attenuated endothelium-dependent relaxation seen in rabbit intrapulmonary vein following chronic nitroglycerine administration. Br. J. Pharmacol. 2005;145:193–202. doi: 10.1038/sj.bjp.0706178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAURSEN J., BOESGAARD S., POULSEN H., ALDERSHVILE J. Nitrate tolerance impairs nitric oxide-mediated vasodilation in vivo. Cardiovasc. Res. 1996;31:814–819. doi: 10.1016/0008-6363(96)00027-2. [DOI] [PubMed] [Google Scholar]
- LIU Y., TERATA K., CHAI Q., LI H., KLEINMAN L., GUTTERMAN D. Peroxynitrite inhibits Ca2+-activated K+ channel activity in smooth muscle of human coronary arterioles. Circ. Res. 2002;91:1070–1076. doi: 10.1161/01.res.0000046003.14031.98. [DOI] [PubMed] [Google Scholar]
- LÜCKHOFF A., BUSSE R. Activators of potassium channels enhance calcium influx into endothelial cells as a consequence of potassium currents. Naunyn Schmiedeberg's Arch. Pharmacol. 1990;342:94–99. doi: 10.1007/BF00178979. [DOI] [PubMed] [Google Scholar]
- MARCHENKO S., SAGE S. Electrical properties of resting and acetylcholine-stimulated endothelium in intact rat aorta. J. Physiol. 1993;462:735–751. doi: 10.1113/jphysiol.1993.sp019579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCHENKO S., SAGE S. Smooth muscle cells affect endothelial membrane potential in rat aorta. Am. J. Physiol. 1994;267:H804–H811. doi: 10.1152/ajpheart.1994.267.2.H804. [DOI] [PubMed] [Google Scholar]
- MARCHENKO S., SAGE S. Calcium-activated potassium channels in the endothelium of intact rat aorta. J. Physiol. 1996;492:53–60. doi: 10.1113/jphysiol.1996.sp021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKLUND S., MARKLUND G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- MARRELLI S., ECKMANN M., HUNTE M. Role of endothelial intermediate conductance KCa channels in cerebral EDHF-mediated dilations. Am. J. Physiol. 2003;285:H1590–H1599. doi: 10.1152/ajpheart.00376.2003. [DOI] [PubMed] [Google Scholar]
- MAYS B.W., FREISCHLAG J.A., EGINTON M.T., CAMBRIA R.A., SEABROOK G.R., TOWNE J.B. Ascorbic acid prevents cigarette smoke injury to endothelium-dependent arterial relaxation. J. Surg. Res. 1999;84:35–39. doi: 10.1006/jsre.1999.5601. [DOI] [PubMed] [Google Scholar]
- MEHRKE G., DAUT J. The electrical response of cultured guinea-pig coronary endothelial cells to endothelium-dependent vasodilators. J. Physiol. 1990;430:251–272. doi: 10.1113/jphysiol.1990.sp018290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MÜNZEL T., LI H., MOLLNAU H., HINK U., MATHEIS E., HARTMANN M., OELZE M., SKATCHKOV M., WARNHOLTZ A., DUNCKER L., MEINERTZ T., FORSTERMANN U. Effects of long-term nitroglycerin treatment on endothelial nitric oxide synthase (NOS III) gene expression, NOS III-mediated superoxide production, and vascular NO bioavailability. Circ. Res. 2000;86:E7–E12. doi: 10.1161/01.res.86.1.e7. [DOI] [PubMed] [Google Scholar]
- MÜNZEL T., SAYEGH H., FREEMAN B., TARPEY M., HARRISON D. Evidence for enhanced vascular superoxide anion production in nitrate tolerance. A novel mechanism underlying tolerance and cross-tolerance. J. Clin. Invest. 1995;95:187–194. doi: 10.1172/JCI117637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKANO Y., KUSAMA N., KAJIKURI J., SUZUKI Y., KANMURA Y., ITOH T. Role of PKC in the attenuation of the cGMP-mediated relaxation of skinned resistance artery smooth muscle seen in glyceryl-trinitrate-tolerant rabbit. Br. J. Pharmacol. 2004;141:391–398. doi: 10.1038/sj.bjp.0705625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NILIUS B., VIANA F., DROOGMANS G. Ion channels in vascular endothelium. Annu. Rev. Physiol. 1997;59:145–170. doi: 10.1146/annurev.physiol.59.1.145. [DOI] [PubMed] [Google Scholar]
- OHASHI M., SATOH K., ITOH T. Acetylcholine-induced membrane potential changes in endothelial cells of rabbit aortic valve. Br. J. Pharmacol. 1999;126:19–26. doi: 10.1038/sj.bjp.0702262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKER J., FARRELL B., FENTON T., COHANIM M., PARKER J. Counter-regulatory responses to continuous and intermittent therapy with nitroglycerin. Circulation. 1991;84:2336–2345. doi: 10.1161/01.cir.84.6.2336. [DOI] [PubMed] [Google Scholar]
- PARUMS D.V., CORDELL J.L., MICKLEM K., HERYET A.R., GATTER K.C., MASON D.Y. JC70: a new monoclonal antibody that detects vascular endothelium associated antigen on routinely processed tissue sections. J. Clin. Pathol. 1990;43:752–757. doi: 10.1136/jcp.43.9.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY P., O'NEILL W. Swelling-activated K fluxes in vascular endothelial cells: volume regulation via K-Cl cotransport and K channels. Am. J. Physiol. 1993;265:C763–C769. doi: 10.1152/ajpcell.1993.265.3.C763. [DOI] [PubMed] [Google Scholar]
- QUIJANO C., HERNANDEZ-SAAVEDRA D., CASTRO L., MCCORD J., FREEMAN B., RADI R. Reaction of peroxynitrite with Mn-superoxide dismutase. Role of the metal center in decomposition kinetics and nitration. J. Biol. Chem. 2001;276:11631–11638. doi: 10.1074/jbc.M009429200. [DOI] [PubMed] [Google Scholar]
- ROSS J., ARMSTEAD W. Differential role of PTK and ERK MAPK in superoxide impairment of KATP and KCa channel cerebrovasodilation. Am. J. Physiol. 2003;285:R149–R154. doi: 10.1152/ajpregu.00003.2003. [DOI] [PubMed] [Google Scholar]
- SAKAI T. Acetylcholine induces Ca-dependent K currents in rabbit endothelial cells. Jpn. J. Pharmacol. 1990;53:235–246. doi: 10.1254/jjp.53.235. [DOI] [PubMed] [Google Scholar]
- SAWA T., AKAIKE T., MAEDA H. Tyrosine nitration by peroxynitrite formed from nitric oxide and superoxide generated by xanthine oxidase. J. Biol. Chem. 2000;275:32467–32474. doi: 10.1074/jbc.M910169199. [DOI] [PubMed] [Google Scholar]
- SCHWENKE D.C., BEHR S.R. Vitamin E combined with selenium inhibits atherosclerosis in hypercholesterolemic rabbits independently of effects on plasma cholesterol concentrations. Circ Res. 1998;83:366–377. doi: 10.1161/01.res.83.4.366. [DOI] [PubMed] [Google Scholar]
- SHARMA N., DAVIS M. Mechanism of substance P-induced hyperpolarization of porcine coronary artery endothelial cells. Am. J. Physiol. 1994;266:H156–H164. doi: 10.1152/ajpheart.1994.266.1.H156. [DOI] [PubMed] [Google Scholar]
- SONG J., DAVIS M. Chloride and cation currents activated by bradykinin in coronary venular endothelial cells. Am. J. Physiol. 1994;267:H2508–H2515. doi: 10.1152/ajpheart.1994.267.6.H2508. [DOI] [PubMed] [Google Scholar]
- VON DER WEID P.Y., BÉNY J. Simultaneous oscillations in the membrane potential of pig coronary artery endothelial and smooth muscle cells. J. Physiol. 1993;471:13–24. doi: 10.1113/jphysiol.1993.sp019888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG X., CHU W., VAN B.C. Potentiation of acetylcholine-induced responses in freshly isolated rabbit aortic endothelial cells. J. Vasc. Res. 1996;33:414–424. doi: 10.1159/000159170. [DOI] [PubMed] [Google Scholar]
- ZHANG H., SPAPEN H., NGUYEN D., BENLABED M., BUURMAN W., VINCENT J. Protective effects of N-acetyl-L-cysteine in endotoxemia. Am. J. Physiol. 1994;266:H1746–H1754. doi: 10.1152/ajpheart.1994.266.5.H1746. [DOI] [PubMed] [Google Scholar]