Abstract
The effect of the KCNQ channel blockers XE991, chromanol 293B and linopirdine, was studied on voltage-dependent K+ currents in smooth muscle cells dissociated freshly from mouse portal vein (mPV) and isometric tension recordings from whole mPV.
Voltage clamp experiments showed XE991 inhibited an outward current in a concentration-dependent manner with an IC50 of 5.8 μM. Block was voltage independent. Chromanol 293B and linopirdine also blocked the voltage-dependent K+ current but were less potent than XE991.
At least two components – a linear (Ilinear) and an outward relaxation (Iout) – contributed to the XE991-sensitive conductance.
XE991-sensitive currents were sustained at all test potentials and XE991 inhibited the enhanced holding current at −60 mV produced by bathing cells in an external solution containing 36 mM KCl.
Current clamp experiments in the perforated-patch configuration showed XE991 and linopirdine depolarised the resting membrane potential and augmented the evoked response in a concentration-dependent manner.
In functional experiments the spontaneous contractile activity of the mPV was increased significantly by XE991 and linopirdine. The stimulatory effect of XE991 was not affected by the presence of 4-AP, glibenclamide nor paxilline.
These data provide evidence for an important role for KCNQ channels in governing cellular excitability in mPV smooth muscle cells.
Keywords: KCNQ, murine, vascular smooth muscle, voltage-dependent potassium channels, XE991
Introduction
The KCNQ (Kv7) subfamily of potassium (K+) channels has been studied extensively in the central nervous system (CNS) and in cardiac cells. The KCNQ1 subunit is almost exclusively localised in atrial and ventricular myocytes, where it is associated with the regulatory subunit encoded by KCNE1 (minK). This combination underlies the slowly activating delayed rectifier (IKs) channel that contributes to the late repolarisation phase of the cardiac action potential (see reviews by Jentsch, 2000; Nerbonne, 2000; Robbins, 2001). Mutations in either KCNQ1 or KCNE1 channel subunits contributes to congenital arrhythmias that are characteristic of long QT syndrome (LQTS) (Ashcroft, 2000). In contrast, KCNQ2, 3, and 5 are expressed predominantly in the CNS and KCNQ4 is localised to auditory nerves (Coetzee et al., 1999; Rennie et al., 2001; Søgaard et al., 2001). Heteromultimers formed from the combination of KCNQ2 and KCNQ3 generate K+ currents sensitive to muscarinic acetylcholine receptor activation (termed M-currents) that contribute to the resting conductance (Wang et al., 1998; Søgaard et al., 2001; Oliver et al., 2003). In contrast to the extensive research on KCNQ channels in the CNS and heart, there are very few studies on these channels in smooth muscle cells (SMCs). To date, KCNQ1 transcripts have been identified in the rat stomach (Ohya et al., 2002a) and the mouse portal vein (mPV, Ohya et al., 2003) but functional information is scarce.
The study of KCNQ channels is hampered by the lack of highly selective blockers. The classical K+ channel blockers tetraethylammonion (TEA) ions and 4-aminopyridine (4-AP) inhibit a large majority of voltage-gated K+ channels, but recombinant KCNQ channel currents showed incomplete block (see reviews by Coetzee et al., 1999; Robbins, 2001). However, chromanol 293B (C293B), linopirdine and its more potent analogue 10,10-bis(4-pyridinyl-methyl)-9(10H)-anthracenone or XE991 (Earl et al., 1998) are considered to be selective blockers of KCNQ channels (Jentsch, 2000; Robbins, 2001). In voltage clamp studies XE991 has been shown not to affect Kv1.2, Kv4.3, eag1, erg1, erg3 nor elk1 channel currents expressed in Xenopus oocytes (Wang et al., 1998). It has been used to study native KCNQ channels in dorsal root ganglion neurones (Passmore et al., 2003), in vestibular cells of the gerbil and mouse (Rennie et al., 2001; Oliver et al., 2003); and recombinant KCNQ channels with and without auxiliary subunits (Wang et al., 1998, 2000; Søgaard et al., 2001).
In our previous study, we showed that mouse portal vein myocytes expressed two forms of KCNQ1 and a component of the gross outward K+ current was blocked by linopirdine (Ohya et al., 2003). The aim of the present study was to extend these preliminary observations by using XE991, a more potent blocker of KCNQ channels, to probe the electrophysiological and functional roles of KCNQ channels in these cells. As the number of studies on KCNQ in SMCs is scarce, experiments with C293B and linopirdine were also undertaken (the structures of these compounds are shown in Figure 1). These data show that KCNQ channels have an important role in defining mPV excitability. Some of these results have been presented in abstract form (Yeung & Greenwood, 2004).
Figure 1.
Structures of KCNQ channel blockers used in the present study.
Methods
Cell preparation
Mouse portal vein (mPV) SMCs were dissociated freshly from 6 to 8-week-old female BALB/c mice (Britton et al., 2002). Animals were killed by cervical dislocation, the portal vein removed and bathed in physiological saline solution (PSS) containing 1 mM Ca2+. Isolated cells were obtained enzymatically by treating the tissue with 100 μM Ca2+ PSS containing 0.3 mg ml−1 protease (type XIV, Sigma, U.K.); then with 0.6 mg ml−1 collagenase (type I, Calbiochem, U.K.), both at 37°C for 6 min. Dispersed cells were kept on ice until required.
Current and voltage recordings
Currents were recorded in the conventional whole-cell patch clamp configuration using electrodes (3–6 MΩ) fabricated from capillary borosilicate glass (Plowden & Thompson, U.K.) using a two-stage electrode puller (PP-830, Narishige, Japan). All experiments were carried out at room temperature (20–22°C). To study the voltage-gated K+ current in isolation, EGTA (5 mM) and ATP (3 mM) were included in the pipette solution and recordings were made using the ruptured patch technique. This rationale has been used in a number of studies (e.g. Evans et al., 1994; Akbarali et al., 1999; Platoshyn et al., 2004) to eliminate any contribution from Ca2+-activated K+ currents, especially of the large conductance type (BKCa) and ATP-sensitive K+ (KATP) currents to the overall outward conductance. The holding potential (VH) of all cells in voltage-clamp was −60 mV and the inhibitory effect of XE991 was assessed after a 5 min equilibration period by stepping to +20 mV for 200 ms every 20 s. The voltage dependence of activation and inactivation of the XE991-sensitive current was determined using a two pulse protocol. Cells were initially stepped to potentials between −100 and +60 mV for 15 s. This was then followed by a 500 ms step to a constant test potential (VT) of 0 mV. This protocol was executed in the absence and presence of XE991 and the XE991-sensitive current defined by subtraction of the latter from the former. Experiments aimed at exploring the contribution of XE991-sensitive channels to the resting conductance employed two different protocols. In the first protocol cells bathed in normal external solution (5 mM KCl) were depolarised to 0 mV for 45 s and the effect of XE991 on currents evoked by this prolonged depolarisation was ascertained. In the second protocol cells were held at −60 mV and bathed in an external solution containing 36 mM KCl. This manoeuvre increased the driving force for K+ flux and therefore augmented any contribution of K+ channels open at the holding potential.
Membrane potential recordings were made using the current clamp plus command setting on the amplifier. Active responses were evoked by the injection of 100–600 pA for 2 or 20 ms. Recordings were made using the internal solution described for the voltage clamp experiments with either the ruptured patch or perforated patch variants of the whole cell recording configuration. For the latter amphotericin (300 μg ml−1) was included in the internal solution and electrical access was monitored by the repetitive application of 10 mV hyperpolarisations until the series resistance was less than 40 MΩ. All recordings were low-pass filtered at 5 kHz and acquired by use of an Axopatch 200B amplifier, Digadata 1322A interface and pClamp (version 9, Axon Instruments, Foster City, U.S.A.). Data were analysed using Clampfit (Axon Instruments), Origin (version 6, Microcal, U.S.A.) and Excel (Microsoft, U.S.A.). All data were obtained from >2 animals. Results were expressed as mean±standard error of means (s.e.m.) and n as the number of cells. Statistical analyses were performed using Student's t-test and were considered significant at the P<0.05 level.
Functional experiments
Portal veins were ligated in situ using 3/0 gauge silk braided suture thread (Pearsells Sutures, Somerset, U.K.) immediately proximal to the porta hepatis and distal to the anastamosis of splenogastric vein and mesenteric vein. Connective tissue and fat were removed by sharp dissection and the tissue placed in a 10 ml organ bath containing Kreb's solution maintained at 37°C and gassed with 95% O2/5% CO2 at a resting tension of 0.1 g. Changes in isometric tension were recorded using BIOPAC Systems Inc. force transducer and AcqKnowledge software (version 3.7). Similar to previous studies (Spencer & Greenwood, 2003; Saleh & Greenwood, 2005) all veins were spontaneously active within minutes of arrangement and maintained rhythmicity for the duration of the experiment. mPVs exhibited a number of different contractile patterns that varied from a single contraction to bursts of contractions. To allow for the data to be quantified, strict thresholds were applied to the measurement of contraction interval and duration. For the purpose of this study a single contraction was defined as an increase in tension from the baseline that was separated from another contraction by a period at the basal tension of at least 1 s. Intercontraction interval was taken as the period between the peaks of two individual contractions. If a tissue exhibited a bursting contraction pattern the interval was taken as the period between the last peak of one contractile event and the first peak of the next event. Contraction duration was taken as the total time that the tension remained above 10% of the maximal contraction.
Solutions
PSS contained (mM): NaCl (125), KCl (5.4), NaHCO3 (15.4), Na2HPO4 (0.33), KH2PO4 (0.34), glucose (10) and HEPES (11), adjusted to pH 7.4 with NaOH. Enzyme solutions were made up with 100 μM Ca2+ PSS. The bathing (external) solution contained (mM): NaCl (126), KCl (5), MgCl2 (1), CaCl2 (0.1), glucose (11), HEPES (10), adjusted to pH 7.2 with NaOH. An external solution containing 5 mM 4-AP was adjusted to pH 7.2 with HCl. The pipette (internal) solution contained (mM): KCl (130), MgCl2 (1), ATP (Na+ salt, 3), GTP (0.1), HEPES (10), EGTA (5) adjusted to pH 7.2 with KOH. The Kreb's solution for the functional experiments contained (mM): NaCl (125), KCl (4.6), CaCl2 (2.5), NaHCO3 (15.4), Na2HPO4 (1), MgSO4 (0.6), glucose (10) constantly aerated by 95% O2/5% CO2. Stock solutions of 100 mM XE991 (diHCl, Tocris, U.K.) and 500 mM 4-AP were made up with distilled water; 100 mM C293B (Tocris, U.K.), 10 mM glibenclamide (RBI, U.S.A.) and 10 mM paxilline (Sigma, U.K.) were made up with dimethyl sulfoxide; 100 mM linopirdine (Sigma, U.K.) was made up with ethanol. All stocks were stored at −20°C until required. Working concentrations were made up with the external solution immediately prior to experimentation and continuously perfused by gravity at a rate of approximately 1–2 ml min−1. All enzymes and salts were purchased from Sigma Chemical Company (U.K.) and VWR International (U.K.).
Results
Effects of XE991 on the total outward delayed rectifier current in single mPV cells
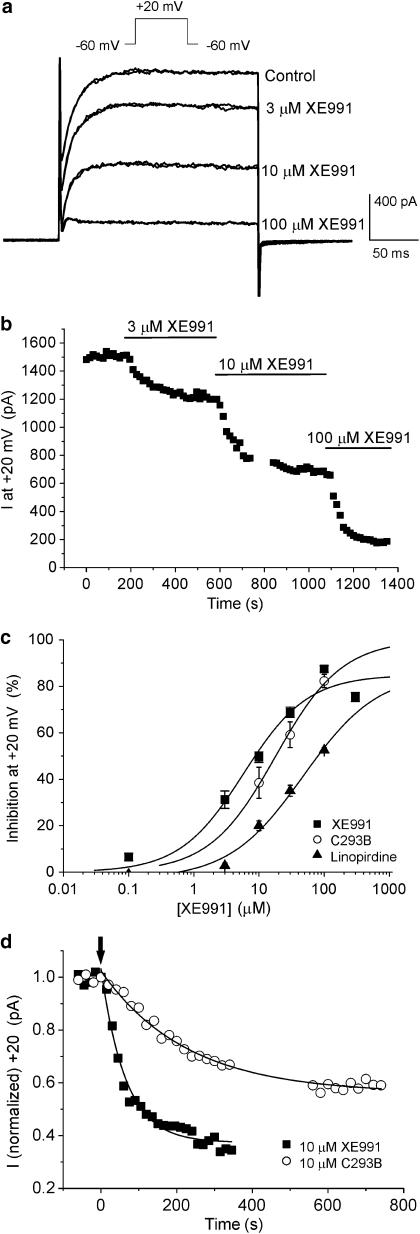
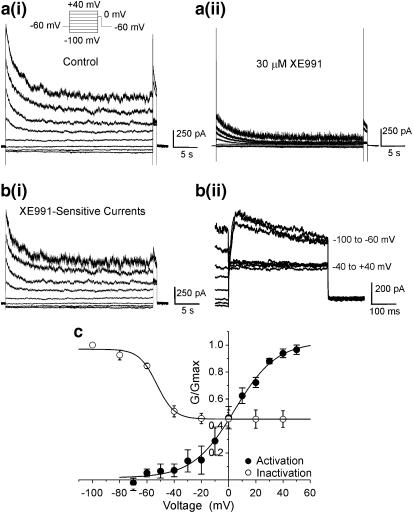
Membrane depolarisation from the holding potential of −60 mV elicited an outward current that activated similar to currents described previously in this cell type (Ohya et al., 2003). The activation threshold of this current was approximately −30 mV with a peak current at +20 of 751±55 pA (n=32). This current was inhibited rapidly by XE991 in a concentration-dependent manner. At 3 μM XE991 block was readily reversible but recovery of the current was progressively less at concentrations of 10 μM and above. For instance after 10 min washout of 10 μM XE991, the peak current at +20 mV was 783.6±146.2 pA compared to mean peak amplitude before XE991 of 1034.1±210.7 pA (n=4, paired data). Figure 2a shows a series of current recordings in the absence and presence of 3, 10 and 100 μM XE991 from a single cell where the outward current was reduced by 20, 55 and 79%, respectively, and the mean concentration–response plot is shown in Figure 2c. Fitting of these data with a logistic function (Response=maximum response/[1+(IC50/concentration)]) gave an IC50 value of 5.8 μM (slope factor (P)=0.9, n=3–12) at a test potential of +20 mV. Inhibition by XE991 was quick, stabilised within a 5-min period and was voltage-independent over the voltage range studied (data not shown). For example, a single concentration of 30 μM XE991 at VT=0 mV reduced the gross outward current amplitude by 68.0±3.6% (n=4) that was similar to the effect at +50 mV (68.2±2.2%, n=4). C293B is another agent, structurally dissimilar to XE991 (see Figure 1), that blocks KCNQ channels. This agent also inhibited the outward current evoked by depolarisation from −60 to +20 mV but the inhibitory effect of this compound was markedly slower than that produced by XE991 (see Figure 2d for comparison). Figure 2c shows the mean inhibitory effect of C293B and fitting of these data by a logistic function yielded an IC50 value of 18 μM (P=0.9, n=4). Figure 2c also contains data on linopirdine taken from Ohya et al. (2003) for comparison. These studies show that the KCNQ channel blockers XE991 and C293B inhibited the gross outward current in murine PV myocytes supporting earlier findings with linopirdine (Ohya et al., 2003).
Figure 2.
XE991 inhibits voltage-dependent outward currents in mPV cells. (a) Example of currents evoked by 200 ms depolarisation from −60 to +20 mV in the absence and when stably inhibited by 3, 10 and 100 μM XE991. At each concentration of XE991 a pair of currents 60 s apart is shown to highlight the completeness of the block. (b) Representative time course of the inhibition produced by XE991. Each time point is the peak current amplitude at +20 mV. Recordings were made every 20 s. Gap in figure represents a period where a current voltage protocol was executed. (c) Concentration–response plots for XE991, C293B (present study) and linopirdine (taken from Ohya et al., 2003) against the gross outward current recorded at +20 mV. Each point is the mean of at least four cells with error bars representing the s.e.m. All plots were fitted with a logistic function. (d) Time course of K+ current inhibition by 10 μM XE991 compared to that produced by 10 μM C293B (○). Arrow denotes start of block and the inhibition produced by both agents was fitted with a single exponential function. Time constants of current decay were 65 and 225 s for XE991 and C293B, respectively.
The gross outward K+ current consists of two components
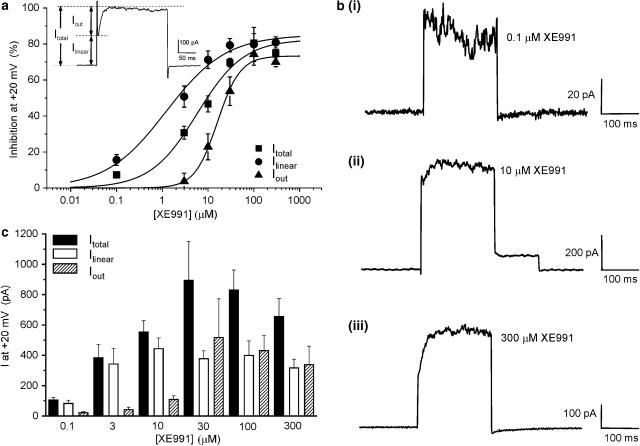
The previous section showed that a definite component of the gross outward current was XE991 sensitive. Closer scrutiny of the voltage-dependent outward current revealed the existence of two distinct components (see inset of Figure 3a). One was linear, time-independent (termed Ilinear) that activated instantaneously upon depolarisation and this was superimposed by a time-dependent component (termed Iout). XE991 inhibited both components but with a markedly different concentration dependence. This was quantified by constructing concentration–response curves for each component. Identical to the concentration–response in Figure 2c, inhibitions for Ilinear and Iout were calculated at +20 mV and data were fitted with a logistic function (Figure 3a). IC50 values and slope values were calculated to be 1.2 μM (P=0.7) and 16.0 μM (P=1.6) for Ilinear and Iout, respectively.
Figure 3.
Dissection of the gross outward current into two components. (a) Concentration–response plot to XE991 at +20 mV for Itotal, Ilinear and Iout. Ilinear can be defined as the instantaneously activated region and Iout as the slower activating component (see inset). Each point is the mean of 3–11 cells. IC50 values were 5.8, 1.2 and 16.0 μM, respectively. (b) XE991-sensitive current recordings at +20 mV determined with different concentrations of XE991. Currents were evoked by 200 ms depolarisations from −60 to +20 mV. Concentrations used were 0.1 (bi), 10 (bii) and 300 μM (biii) XE991. (c) Bar chart illustrating XE991-sensitive current amplitudes of the Itotal, Ilinear and Iout components.
The correlation between the XE991 concentration, Ilinear and Iout could be seen more clearly in the XE991-sensitive current recordings (Figure 3b). With 0.1 μM XE991 only a small Ilinear amplitude was recorded (Figure 3bi). No appreciable Iout was observed. Increasing the concentration of XE991 resulted in a XE991-sensitive current with a larger Ilinear and Iout (Figures 3bii and biii). Currents sensitive to concentrations of XE991 greater than 10 μM exhibited a progressively large Iout. The bar chart (Figure 3c) summarises current amplitudes sensitive to different concentrations of XE991 at a single test potential of +20 mV for Itotal, Ilinear and Iout. With concentrations of XE991 between 3 and 300 μM XE991, the amplitude of Ilinear was fairly consistent while Iout increased in a concentration-dependent manner (Figure 3). At 100 and 300 μM XE991, Ilinear and Iout contributed equally to the XE991-sensitive current. Identical analyses of C293B-inhibited and C293B-sensitive currents were performed. Similar to XE991, C293B was a more effective blocker of the linear component of the gross outward current (IC50 and slope values were 15.5 μM and 0.8) compared to the time-dependent current (IC50 and slope values were 62.8 μM and 0.7). Collectively, these data revealed that the gross outward K+ current in mPV cells sensitive to the KCNQ blockers XE991 and C293B was made up of two K+ distinct components.
Effects of XE991 in the presence of 4-AP
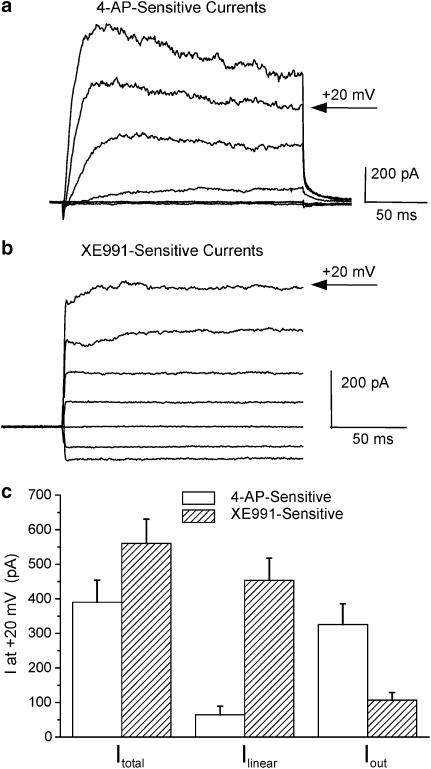
While XE991 and C293B are considered to be selective blockers of KCNQ channels it is possible that the effects of these compounds may be due to inhibition of other voltage-dependent K+ currents (Kv). Consequently, further experiments were performed in the presence of 5 mM 4-AP that was sufficiently high to block most 4-AP-sensitive voltage-gated K+ channels (Coetzee et al., 1999). Application of 5 mM 4-AP inhibited the gross outward current by 61.9±5.6% (n=5); but further inhibition was observed with 10 μM XE991 in the continual presence of 4-AP. This further reduction in current amplitude by XE991 when represented as a ratio of 4-AP-inhibited currents equated to 54.7±6.6% (n=4, paired data), which was identical to cells not treated with 4-AP. Consequently, XE991 reduced the voltage-dependent outward current in PV myocytes in the absence and presence of 4-AP. While both 4-AP and XE991 inhibited the gross outward current there were marked difference in the effects of each agent on the individual components described in the previous section. Figure 4a shows that the 4-AP-sensitive current activated relatively slowly and in a voltage-dependent manner whereas the current sensitive to 10 μM XE991 was time independent except at very positive potentials (see Figure 4b). At +20 mV the 4-AP-sensitive current was composed of a very small and variable Ilinear (mean amplitude was 64±25 pA, n=11) and a large time-dependent current (325±60 pA). This contrasted with the XE991-sensitive current at the same potential that was composed of a 453±64 pA linear component and 107±22 pA time-dependent component (n=12). Consequently, 4-AP and XE991 affected kinetically different currents in mPV myocytes.
Figure 4.
Characteristics of 4-AP- and XE991-sensitive currents. (a) Example of currents evoked by 200 ms depolarisations from −60 mV to various test potentials from −80 to +40 mV that were sensitive to 5 mM 4-AP. (b) Current recordings sensitive to 10 μM XE991. Traces were truncated to the initial 200 ms of depolarising test steps for comparison with (a). (c) Bar chart illustrating the amplitudes of the Itotal, Ilinear and Iout components of the 4-AP- and XE991-sensitive currents.
Kinetic properties of the XE991-sensitive current
The voltage-dependent activation and inactivation of XE991-sensitive currents were determined using voltage protocols shown in Figures 2a and 5a. For activation, subtracted current amplitudes were measured in a 200 ms window from VH to various test potentials between −100 and +60 mV in the absence and presence of a submaximal concentration of XE991 (30 μM, Figure 5c). Subtracted data were fitted by a Boltzmann function to give a V1/2 of activation of 7±3 mV with a slope value of 14±2 mV (n=4). The voltage dependence of XE991-sensitive current inactivation was quantified by a standard two-pulse protocol (Figure 5ai). Current availability was assessed at a single VT of 0 mV after a 15 s prepulse to various potentials in the absence and presence of 30 μM XE991 (Figure 5a). The subtracted current amplitude at VT was then plotted against the prepotential and fitted by the Boltzmann function (Figure 5c). By this method the V1/2 of inactivation of the XE991-sensitive current cells was calculated to be −54.3±2.1 mV (n=4). It is obvious from Figures 5b and c that the XE991-sensitive current failed to inactivate completely and that the XE991-sensitive current was composed of a transient and a sustained component of roughly equal amplitude. This is consistent with this concentration of XE991 (30 μM) blocking both Ilinear and Iout maximally (Figure 3a). The reduction of the peak current to a sustained level was best fitted by a double exponential with mean tau values of 274±57 and 2962±1048 ms (n=7). These data show that the XE991-sensitive current in mPV myocytes activated and inactivated in a voltage-dependent manner. Prolonged depolarisations (⩾15 s) revealed that the XE991-sensitive current was sustained at all test potentials.
Figure 5.
Inactivation properties of the XE991-sensitive current. (a) Inactivation of the XE991-sensitive current was investigated by a two-pulse protocol described in the inset. Currents were evoked every 60 s. Examples of currents evoked by this protocol in the absence (ai) and presence (aii) of 30 μM XE991. XE991-sensitive recordings from the same cell as (a) are shown in panel (bi). An enlargement of the XE991-sensitive currents at VT (0 mV) is shown in (bii). Panel c shows the mean data from experiments typified in (a). Vertical axis is the mean amplitude of the XE991-sensitive current at VT normalised to the maximum current at VT. Horizontal axis is the prepotential (mV). Data points were fitted with a Boltzmann function to give V1/2 inact=−54.3±2.1 mV (n=4). Activation data were normalised peak amplitudes at various prepotentials. Data were fitted with a Boltzmann function to give V1/2=7.1±2.7 mV (n=4).
KCNQ channels contribute to the RMP
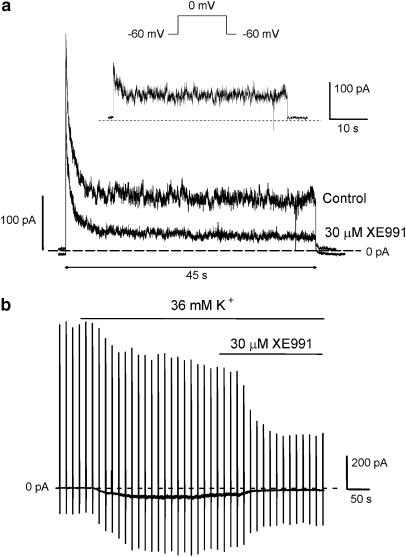
KCNQ channels contribute to setting the resting membrane potential (RMP) in neurones (Wang et al., 1998; Søgaard et al., 2001; Oliver et al., 2003). The possibility that KCNQ channels may have a similar role in vascular SMCs was therefore investigated. The above data showed that a significant amount of current was still available at the end of a 15 s depolarising test step. We extended the duration of the test pulse to insure that the apparent lack of full inactivation was not due to the 15 s pulse being too short to force the channels to inactivate fully. Figure 6a shows an example of currents evoked by a 45 s depolarisation to 0 mV under control conditions and when stably inhibited by 30 μM XE991. In four cells tested, the peak and sustained components were effectively reduced by 45.9±7.8 and 82.6±6.0% (P<0.05), respectively. The mean amplitudes of the XE991-sensitive current was 232±94 pA for the transient current and 103±32 pA for the sustained current. In all cases, the peak amplitude was twice that of the sustained current. The comparatively larger inhibition of the sustained component further supports initial experiments where a linear conductance was found, which had a greater sensitivity to XE991 than either Itotal or Iout. Consequently, there was a significant level of time-independent XE991-sensitive current in mPV myocytes that would contribute to the resting conductance. This was investigated further by a second protocol where mPV cells were held at −60 mV and the resting K+ conductance was augmented by raising the [K+] of the external solution ([K+]ex) to 36 mM similar to Evans et al. (1996). Figure 5b shows that this manoeuvre increased the amplitude of IH at −60 mV (mean change was from −2±2 to −73±16 pA). Application of 30 μM XE991 in the continual presence of 36 mM [K+]ex resulted in a marked reversal of IH (mean reduction of the enhanced IH was 80.0±5.2%, n=4, Figure 6b). The inhibition of the enhanced IH was fully reversible on washout of XE991 (data not shown). This degree of inhibition was identical to that observed for the block of the sustained component at the end of a 45 s depolarisation (80.3±5.2%, n=5) described above. Similar effects were obtained with 3 μM XE991 that inhibited the holding current at −60mV in a bathing solution containing 36 mM K+ by 60±1% (n=3). These data support a role of KCNQ channels in contributing to the resting conductance and infer that alteration of this conductance would have important effects on the RMP.
Figure 6.
Evidence that XE991-sensitive currents contribute to resting conductance. (a) Example of currents evoked by a 45 s depolarisation from −60 to 0 mV in the absence and presence of 30 μM XE991. Inset shows the XE991-sensitive current evoked by this protocol and highlights the existence of transient and sustained components. (b) Long-term trace showing the effect of 30 μM XE991 on currents recorded from mPV myocytes held at −60 mV bathed in an external solution containing 36 mM KCl. Deflections represent membrane steps to +20 mV every 15 s.
Effects of XE991 in current clamp experiments
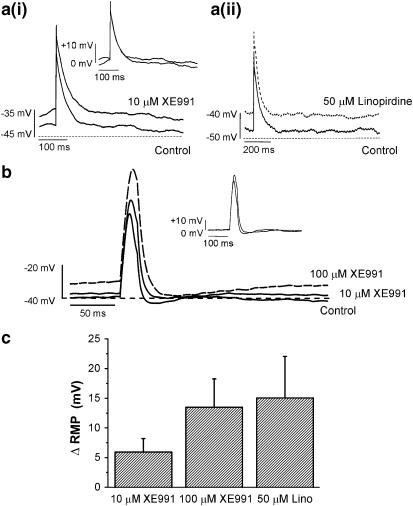
The voltage clamp data provide evidence that the XE991-sensitive conductance could contribute to the resting membrane potential so current clamp experiments were undertaken to assess this possibility. Initial experiments were performed using the perforated patch configuration to maintain a more physiological intracellular environment that also allowed intracellular [Ca2+] to rise. Under these conditions the RMP was observed to be −46±2 mV (n=15) and depolarising current injections (2–20 ms, 100–200 pA) evoked action potentials (AP) of 49±3 mV (n=11, Figure 7). In the presence of 10 μM XE991, a small depolarisation of the membrane potential was recorded (ΔRMP was 6±3 mV, n=9, paired data). A higher concentration (100 μM) of XE991 was used to determine any concentration-dependent changes in the action potential. With this concentration, a larger change in the RMP was observed (ΔRMP was 13±5 mV, n=4, paired data). Linopirdine (50 μM, a concentration close to the IC50 determined by Ohya et al., 2003) also depolarised the RMP by 9±5 mV, n=4). XE991 also augmented the amplitude of the evoked AP in a concentration dependent manner (mean increase in amplitude produced by 10 and 100 μM XE991 was 18±12 and 30.3±11.0 mV, respectively, see Figure 7a). With brief injections of current (2 ms) 10 μM XE991 had no effect on the action potential duration at 90 or 20% of maximum amplitude (APD90% or APD20%) and 100 μM had a small but significant effect on APD20% (n=5). However, with 20 ms current injections the APD20% was marginally broadened with 10 μM XE991 (change=11%) that became more significant with 100 μM XE991 (change=83%).
Figure 7.
Effect of XE991 on membrane potential recordings in current clamp mode. (a) Examples of action potentials elicited by 2 ms injection of current in the absence and presence of 10 μM XE991 (ai) and 50 μM linopirdine (aii). Dashed lines represent −35 mV (ai) and −50 mV (aii). Inset (ai) illustrates scaled potentials in the absence and presence of XE991. (b) Representative recordings under control conditions and in the presence of 10 and 100 μM XE991. Dashed line represents −40 mV. Voltage responses were evoked by a brief current injection (20 ms, 600 pA). Inset represents scaled responses to control and 10 μM XE991. (c) Bar chart showing depolarising changes in the RMP of perforated cells when in continual contact with XE991 (10 and 100 μM) and linopirdine (50 μM). All recordings were made using the perforated patch variant of the whole cell patch clamp technique.
Further experiments were undertaken using the ruptured patch-recording configuration similar to the voltage clamp experiments. This technique allowed the calcium buffer EGTA to flood the cell interior and prohibit the activation of Ca2+-dependent conductances. As such this configuration favoured an influence from voltage-dependent, Ca-independent conductances. Notable differences between the two configurations included a relatively more hyperpolarised RMP with ruptured patch recordings (mean was −61±2 mV, n=9); a larger current (200–600 pA) injection that was required to elicit an AP that was markedly smaller (39.8±2.8 mV, n=9) than recorded with the perforated patch configuration. Again XE991 depolarised the RMP by 13±2 mV (10 μM) and 36±8 mV (100 μM, P<0.05, n=4, paired data). The AP amplitude was also increased significantly by 81±21 and 124±17% for 10 and 100 μM XE991, respectively. Overall these data support a role for KCNQ channels in the maintenance of the RMP in mPV SMCs.
Functional experiments
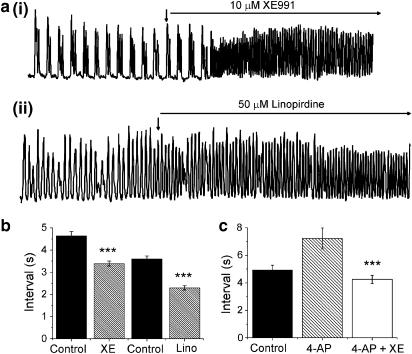
Data from previous sections suggested that in mPV SMCs an XE991-sensitive current contributed to the RMP and maintained the cell in a less excitable state. This putative functional role of XE991-sensitive channels was assessed in isometric tension studies. Under control conditions, all murine portal veins exhibited spontaneous contractile activity that exhibited various patterns (see Figure 8a and Spencer & Greenwood, 2003). Regardless of the nature of the contractions the addition of 10 μM XE991 (Figure 8a(i)) and 50 μM linopirdine (Figure 8a(ii)) increased the frequency of contractions. In some tissues the large increase in frequency resulted in a tonic contraction (data not shown). Figure 8b shows the interval between contractions in the presence of XE991 and linopirdine (hatched bars) was significantly (P<0.05) less than the respective controls. These equated to respective reductions of 28 and 36%. Similar changes (P<0.05) in the duration of the contractions were also observed (data not shown). Under voltage clamp conditions, XE991 was still able to inhibit the gross outward current in the presence of 5 mM 4-AP. It was therefore of interest to examine the contractile responses of the portal vein tissue under similar conditions. 4-AP was allowed to equilibrate with the tissue for approximately 10 min prior to addition of XE991. The bar chart in Figure 8c illustrates the marginally prolonged interval between contractions in 5 mM4-AP. However, application of 10 μM XE991 in the continual presence of 4-AP significantly (P<0.05) reduced intercontraction interval from 7.2±0.8 to 4.3±0.3 s (n=8, P<0.001). This represented a 38% change similar to that produced in the absence of 4-AP. A final series of experiments investigated whether XE991 could modify contractile activity when KATP and BKCa were eliminated. This was achieved by application of KATP (glibenclamide, 10 μM) and BKCa (paxilline, 1 μM) channel blockers. These were equilibrated with tissue for approximately 10 min prior to application of 10 μM XE991. In the presence of paxilline and glibenclamide the mean intercontraction interval was 8.13±0.9 s (n=5) and this was reduced considerably by subsequent application of 10 μM XE991 (mean interval was 3.13±0.11 s, n=5, P<0.001 paired data). These data showed that XE991 significantly increased spontaneous contractility even in the presence of 4-AP, glibenclamide and paxilline at concentrations that would block Kv, KATP and BKCa channel currents.
Figure 8.
XE991 increases the excitability of whole hepatic portal veins. (a) Examples of isometric tension recordings in the absence and presence of (i) 10 μM XE991 and (ii) 50 μM linopirdine. Upward deflections represent spontaneous increase in tone. XE991 was applied at the downward arrow and was present for the remainder of the trace. (b) Bar chart showing the mean contraction interval in the absence (solid bars) and presence of two KCNQ channel blockers (hatched bars). The 10 μM XE991 (n=5 tissues) and 50 μM linopirdine (n=3 tissues) both significantly (P<0.001) reduced the interval. (c) Application of 10 μM XE991 in the presence of 5 mM4-AP still resulted in a reduction of the mean contraction interval (***P<0.001).
Discussion
The aim of the present study was to extend our previous findings that myocytes from murine PV express KCNQ channels (Ohya et al., 2003) and to establish the impact of these channels on mPV excitability. In the initial study by Ohya et al. (2003) linopirdine was used to probe for a contribution of KCNQ channels to voltage-dependent K+ current. To give insight into the functional role of KCNQ channels we have utilised the more potent analogue of linopirdine, XE991 as well as an established blocker of IKs (C293B) in concert with linopirdine. Data obtained from experiments with all three agents add weight to our original findings (Ohya et al., 2003) and suggest that KCNQ channels are a crucial determinant of the resting membrane conductance and therefore, the excitability of the smooth muscle.
Effects of KCNQ channel blockers
Consistent with data from other studies the present investigation showed that XE991 was a more potent inhibitor of K+ currents in the mouse PV than C293B and linopirdine with respective IC50 values for inhibition of the gross outward current of 5.8, 18 and 48 μM (from Ohya et al., 2003). This potency profile was similar to other studies (e.g. Passmore et al., 2003; Romero et al., 2004). Scrutiny of the XE991- and C293B-sensitive currents revealed the existence of two distinct components of the outward current (termed Ilinear and Iout) that had markedly different sensitivities to the two KCNQ channel blockers. For instance the IC50 for inhibition of Ilinear by XE991 was 1.2 μM compared to an IC50 value against Iout of 16 μM. Similar effects are apparent for C293B in guinea pig PV myocytes upon close examination of the data presented by Karle et al. (2002). With long (>15 s) pulses Ilinear was manifest as a maintained, noninactivating current whereas Iout decayed rapidly. Evans et al. (1996) and Osipenko et al. (1997) described a K+ current with similar characteristics in rabbit pulmonary artery SMCs. This current was well maintained after an initial decay for depolarisations lasting up to 10 min and was relatively insensitive to 4-AP, a standard blocker of conventional voltage-dependent K+ channels. Similarly, the effects of XE991 were still observed in the presence of 5 mM 4-AP although some attenuation of the current size was recorded.
Consistent with the XE991-sensitive current being a crucial component of the cell resting conductance current clamp recordings showed that XE991 and linopirdine depolarised PV myocytes significantly. Further evidence for a strong role of KCNQ channels in determining smooth muscle excitability was provided by functional experiments where XE991 and linopirdine increased the frequency of spontaneous contractions markedly even in the presence of 4-AP, paxilline and glibenclamide. It is worth noting that 5 mM 4-AP alone had a significant effect on PV activity but this was manifest as an increase in the amplitude and duration of individual contractions whereas the KCNQ channel blockers primarily reduced the intercontraction interval. These effects were consistent with 4-AP inhibiting an ion channel that governed membrane potential repolarisation after action potential discharge versus XE991 blocking a resting conductance. A number of different voltage-dependent K+ channel genes are expressed in vascular SMCs (see Fountain et al., 2004 for summary) and the expression profile appears to rely on the vessel under study. For instance, KCNA expression is significantly higher in murine resistance arteries than conduit arteries (Fountain et al., 2004). The importance of voltage-dependent K+ conductances in regulating vascular smooth muscle cell excitability is well accepted but the relative importance of different isoforms again seems to be vessel specific. Until recently KCNQ gene expression had not been shown in vascular SMCs. However, evidence for expression of full-length KCNQ1 and a novel C-terminal truncated variant was determined by PCR and immuno-cytochemistry in mPV myocytes (Ohya et al., 2003). The present work extends upon these molecular data and provides evidence for a functional role of this conductance in this vessel. It is worth stressing that this study focused exclusively upon the role of KCNQ channels. Consequently, no attempt was made to dissect out the roles of other voltage-dependent K+ channels such as Kv1.2 or Kv1.5 (KCNA2 and 5) that form a component of the voltage-dependent K+ current in rabbit PV myocytes (Kerr et al., 2001). Moreover, the effects of XE991, linopirdine and C293B have not been screened against all voltage-dependent K+ channels. Regardless of these points the fact that three blockers of KCNQ channel had similar effects on spontaneous contractility, on ion currents and membrane potential and the effects of XE991 were not ablated by preapplication of 5 mM 4-AP reinforced the postulate that noninactivating KCNQ channels were crucial determinants of smooth muscle excitability in mPV.
It is worth highlighting that while XE991 still inhibited voltage-dependent K+ currents in mPV myocytes in the presence of 5 mM 4-AP, this agent reduced the absolute amplitude of the XE991-sensitive current. As it is generally accepted that 4-AP does not inhibit KCNQ channels this observation raises the spectre that XE991 was not a selective inhibitor of KCNQ channels especially at concentrations greater than 10 μM. However, the similarity of the effects produced by C293B and linopirdine to those produced by XE991 suggests that we need not be so quick to condemn this compound especially as the linear component of the XE991-sensitive current was relative insensitive to 4-AP. An alternative explanation of the data in the present study is that the KCNQ channels in mPV myocytes were sensitive to the relatively high concentration of 4-AP used. To date a rigorous examination of the sensitivity of KCNQ1 channels to 4-AP has not been performed. In fact, preliminary studies showed that the KCNQ1b isoform was inhibited considerably by 5 mM 4-AP (G.P. Sergeant, unpublished observations). Interestingly, the noninactivating K+ current proposed to contribute to the resting membrane potential in rabbit pulmonary artery myocytes was also partially sensitive (∼50%) to 5 mM 4-AP (Evans et al., 1996). Future experiments need to focus on the 4-AP sensitivity of heterologously expressed KCNQ isoforms. In addition, accessory proteins have been shown to alter the pharmacological sensitivity of KCNQ channels to blocking agents such as XE991 and a similar effect may occur for 4-AP. The similarity of the effects of all three KCNQ channel blockers and discordance with those effects produced by 4-AP suggest that these compounds at low concentrations are effective probes for KCNQ channels. Accepting the caveats raised above the use of these agents have revealed a sustained KCNQ-dependent current in murine portal vein myocytes that seems to contribute to the resting conductance.
Molecular composition of the XE991-sensitive K+ channel in mPV myocytes
While KCNQ channel blockers used in this study had an undeniable effect on voltage-dependent K+ currents and PV contractility, the concentration of XE991, linopirdine and C293B required to inhibit the gross outward current were higher than required to block heterologously expressed KCNQ channels (e.g. 0.75 μM, Wang et al., 1998) and native neuronal M-currents (0.26 μM, Passmore et al., 2003; Romero et al., 2004). However, close analysis of the effects of XE991 and C293B in the present study revealed that a sustained component of the outward current (Ilinear) was blocked with a pharmacological sensitivity close to previously published data. Moreover, coexpression of KCNQ1 with a number of auxiliary subunits of the KCNE gene family reduced the pharmacological sensitivity of the KCNQ channel to blockers such as XE991 (see mini review by McCrossan & Abbott, 2004). The combination of KCNQ1 with either KCNE1 or KCNE5 resulted in currents that were blocked by XE991 with an IC50 of between 1 and 10 μM (e.g. Wang et al., 2000; Angelo et al., 2002). In addition, a recent report by Heitzmann et al. (2004) revealed that association with KCNE1-3 altered the pharmacological sensitivity of KCNQ1 channels to C293B. The IC50 values for KCNQ1+KCNE1, KCNQ1+KCNE2 and KCNQ1+ KCNE3 were 9.8, 0.4 and 4.3 μM, respectively. Therefore, the two components of the XE991-sensitive current in mPV myocytes could reflect different combinations of KCNQ1 with KCNE proteins. It is also possible that the truncated KCNQ1 isoform discovered in murine PV myocytes (Ohya et al., 2003) may contribute to the XE991-sensitve conductance.
The biophysical properties of KCNQ channels are also affected by expression with KCNE proteins and murine PV myocytes express KCNE1 and KCNE3 strongly and KCNE2 to some extent (Ohya et al., 2002a). K+ currents generated by KCNQ1 in combination with either KCNE1 or KCNE5 activated considerably slower than KCNQ1 expressed alone (Barhanin et al., 1996; Sanguinetti et al., 1996) and the slight inactivation inherent to KCNQ1 was lost. However, coexpression of KCNQ1 with KCNE3 resulted in a channel that activated almost instantaneously and had a Ilinear–voltage relationship (Schroeder et al., 2000). Similar time-independent K+ currents were generated by the coexpression of KCNQ1 and KCNE2 (Tinel et al., 2000). Consequently, the time-independent K+ current identified with lower concentrations of XE991 and C293B may represent the combination of KCNQ1 with either KCNE2 or KCNE3. The inactivating current that is apparent at higher concentrations of KCNQ channel blocker may reflect a combination of KCNQ1 and KCNE1 or may be due to a contribution of the C-terminal truncated variant of KCNQ1 identified by Ohya et al. (2003) that exhibits considerable inactivation. KCNQ1 channels do exhibit inactivation (Tristani-Firouzi & Sanguinetti, 1998) but not to the extent observed for the XE991-sensitive current. Alternatively, the differences in kinetics may reflect subtle changes in the KCNQ isoform expressed as Seebohm et al. (2001) showed that chimeric proteins formed from the insertion of the S6 domain of KCNQ2 into a KCNQ1 background enhanced the intrinsic inactivation resulting in rapidly declining K+ currents.
There has been much focus on interactions between KCNQ1 and KCNE subunits but other families of K+ channel auxiliary subunits such as the KChIPs (K+ Channel Interacting Proteins) and KChAPs (K+ Channel-Associated Proteins) should not be dismissed although an interaction between members of these protein families has not been shown yet. Furthermore, Rennie et al. (2001) reported that a subset of gerbil vestibular neurones exhibited a K+ current blocked by XE991 with kinetics very similar to that described in the present study. These authors proposed that the XE991-sensitive current in vestibular neurones was generated by either a novel KCNQ isoform or combination of existing KCNQ proteins with ERG-encoded gene products similar to the situation in some neurones (Selyanko et al., 1999). This latter scenario is possible as mPV myocytes express two variants of ERG1 (Ohya et al., 2002b) and recent coimmunoprecipitation experiments show that HERG and KCNQ1 gene products interact physically (Ehrlich et al., 2004). If so, this would represent a novel heteromer with α-subunits from different subfamilies.
Vascular importance of KCNQ channels
Murine portal veins are spontaneously active (Spencer & Greenwood, 2003; Saleh & Greenwood, 2005), driven by Ca2+ influx through voltage-dependent, dihydropyridine-sensitive Ca2+ channels (Spencer & Greenwood, 2003; Saleh & Greenwood, 2005). As such the PV represents an atypical blood vessel and it's distinctive inherent rhythmicity reflects it's unique physiological position. However, a noninactivating, 4-AP-resistant K+ current with characteristics similar to those described for the XE991 sensitive in the present study is present in myocytes from pulmonary arteries (Evans et al., 1996). Therefore, it is possible that KCNQ channels may govern cellular excitability in a number of blood vessels. As there are a number of hereditary mutations in KCNQ1 and KCNE isoforms that underlie the majority of congenital arrhythmias then the identification of a KCNQ contribution to the resting membrane potential has profound implications.
Acknowledgments
This work was supported by the British Heart Foundation (project Grant no. 15747). We thank Dr G. Sergeant, Dundalk Institute of Technology, for the unpublished data on KCNQ1b.
Abbreviations
- 4-AP
4-aminopyridine
- C293B
chromanol 293B
- Ilinear
linear current
- Iout
time-dependent outward current
- Itotal
total outward K+ current
- mPV
mouse portal vein
- SMC
smooth muscle cell
- VH
holding potential
- VT
test potential
- XE991, 10
10-bis(4-pyridinyl-methyl)-9(10H)-anthracenone
References
- AKBARALI H.I., THATTE H., HE X.D., GILES W.R., GOYAL R.K. Role of HERG-like K+ currents in opossum esophageal circular smooth muscle. Am. J. Physiol. (Cell Physiol.) 1999;277:C1284–C1290. doi: 10.1152/ajpcell.1999.277.6.C1284. [DOI] [PubMed] [Google Scholar]
- ANGELO K., JESPERSEN T., GRUNNET M., NIELSEN M.S., KLAERKE D.A., OLESEON S.-P. KCNE5 induces time- and voltage-dependent modulation of the KCNQ1 current. Biophys. J. 2002;83:1997–2006. doi: 10.1016/S0006-3495(02)73961-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASHCROFT F.M. Ion Channels and Disease. London: Academic Press; 2000. [Google Scholar]
- BARHANIN J., LESAGE F., GUILLEMARE E., FINK M., LAZDUNSKI M., ROMEY G. KvLQT1 and IsK (minK) proteins associate to form the IKs cardiac potassium current. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- BRITTON F.C., OHYA S., HOROWITZ B., GREENWOOD I.A. Comparison of the properties of CLCA1 generated currents and ICl(Ca) in murine portal vein smooth muscle cells. J. Physiol. 2002;539:107–117. doi: 10.1113/jphysiol.2001.013170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COETZEE W.A., AMARILLO Y., CHIU J., CHOW A., LAU D., MCCORMACK T., MORENO H., NADAL M.S., OZAITA A., POUNTNEY D., SAGANICH M., VEGA-SAENZ DE MIERA E., RUDY B. Molecular diversity of K+ channels. Ann. NY Acad. Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- EARL R.A., ZACZEK R., TELEHA C.A., FISHER B.N., MACIAG C.M., MARYNOWSKI M.E., LOGUE A.R., TAM W., TINKER W.J., HUANG S.-M., CHORVAT R.J. 2-Fluoro-4-pyridinylmethyl analogues of linopirdine as orally active acetylcholine release enhancing agents with good efficacy and duration of action. J. Med. Chem. 1998;41:4615–4622. doi: 10.1021/jm9803424. [DOI] [PubMed] [Google Scholar]
- EHRLICH J.R., POURRIER M., WEERAPURA M., ETHIER N., MARMABACHI A.M., HÉBERT T.E., NATTEL S. KvLQT modulates the distribution and biophysical properties of HERG – a novel α-subunit interaction between delayed rectifier currents. J. Biol. Chem. 2004;279:1233–1241. doi: 10.1074/jbc.M309087200. [DOI] [PubMed] [Google Scholar]
- EVANS A.M., CLAPP L.H., GURNEY A.M. Augmentation by intracellular ATP of the delayed rectifier current independently of the glibenclamide-sensitive K-current in rabbit arterial myocytes. Brit. J. Pharmacol. 1994;111:972–974. doi: 10.1111/j.1476-5381.1994.tb14836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS A.M., OSIPENKO O.N., GURNEY A.M. Properties of a novel K+ current that is active at resting potential in rabbit pulmonary artery smooth muscle cells. J. Physiol. 1996;496:407–420. doi: 10.1113/jphysiol.1996.sp021694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOUNTAIN S.J., CHEONG A., FLEMMING R., MAIR L., SIVAPRASADARAO A., BEECH D.J. Functional up-regulation of KCNA gene family expression in murine mesenteric resistance artery smooth muscle. J. Physiol. 2004;556:29–42. doi: 10.1113/jphysiol.2003.058594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEITZMANN D., GRAHAMMER F., Von HAHN T., SCHMITT-GRÄFF A., ROMEO E., NITSCHKE R., GERLACH U., LANG H.J., VERRY F., BARHANIN J., WARTH R. Heteromeric KCNE2/KCNQ1 potassium channels in the luminal membrane of gastric parietal cells. J. Physiol. 2004;561:547–557. doi: 10.1113/jphysiol.2004.075168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENTSCH T.J. Neuronal potassium KCNQ channels: physiology and role in disease. Nat. Rev. Neurosci. 2000;1:21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- KARLE C.A., BAUER A., WERETKA S., ZITRON E., ABUSHI A., KREYE V.A.W., SCHOELS W. Vascular effects of class-III antiarrhythmic drugs: chromanol 293B, but not dofetilide blocks the smooth muscle delayed rectifier K+ channel. Basic Res. Cardiol. 2002;97:17–25. doi: 10.1007/s395-002-8383-9. [DOI] [PubMed] [Google Scholar]
- KERR P.M., CLEMENT-CHOMIENNE O., THORNLOE K.S., CHEN T.T., ISHII K., SONTAG D.P., WALSH M.P., COLE W.C. Heteromultimeric Kv1.2–Kv1.5 channels underlie 4-aminopyridine-sensitive delayed rectifier K+ currents of rabbit vascular myocytes. Circ. Res. 2001;89:1038–1044. doi: 10.1161/hh2301.100803. [DOI] [PubMed] [Google Scholar]
- MCCROSSAN Z.A., ABBOTT G.W. The MinK-related peptides. Neuropharmacol. 2004;47:787–821. doi: 10.1016/j.neuropharm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- NERBONNE J.M. Molecular basis of function voltage-gated K+ channel diversity in the mammalian myocardium. J. Physiol. 2000;525:285–298. doi: 10.1111/j.1469-7793.2000.t01-1-00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHYA S., ASAKURA K., MURAKI K., WATANABE M., IMAIZUMI Y. Molecular and functional expression of ERG, KCNQ, and KCNE subtypes in rat stomach smooth muscle. Am. J. Physiol. (Gastro. Liv. Physiol.) 2002a;282:277–287. doi: 10.1152/ajpgi.00200.2001. [DOI] [PubMed] [Google Scholar]
- OHYA S., HOROWITZ B., GREENWOOD I.A. Functional and molecular identification of ERG channels in murine portal vein myocytes. Am. J. Physiol. (Cell Physiol.) 2002b;283:C866–C877. doi: 10.1152/ajpcell.00099.2002. [DOI] [PubMed] [Google Scholar]
- OHYA S., SERGEANT G.P., GREENWOOD I.A., HOROWITZ B. Molecular variants of KCNQ channels expressed in murine portal vein myocytes. Circ. Res. 2003;92:1016–1023. doi: 10.1161/01.RES.0000070880.20955.F4. [DOI] [PubMed] [Google Scholar]
- OLIVER D., KNIPPER M., DERST C., FAKLER B. Resting rotential and submembrane calcium concentration of inner hair cells in the isolated mouse cochlea are set by KCNQ-type potassium channels. J. Neurosci. 2003;23:2141–2149. doi: 10.1523/JNEUROSCI.23-06-02141.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSIPENKO O.N., EVANS A.M., GURNEY A.M. Regulation of the resting potential of rabbit pulmonary artery myocytes by a low threshold, O2-sensing potassium current. Br. J. Pharmacol. 1997;120:1461–1470. doi: 10.1038/sj.bjp.0701075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASSMORE G.M., SELYANKO A.A., MISTRY M., AL-QATARI M., MARSH S.J., MATTHEWS E.A., DICKENSON A.H., BROWN T.A., BURBIDGE S.A., MAIN M., BROWN D.A. KCNQ/M currents in sensory neurons: significance for pain therapy. J. Neurosci. 2003;23:7227–7236. doi: 10.1523/JNEUROSCI.23-18-07227.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLATOSHYN O., REMILLARD C.V., FANTOZZI I., MANDEGAR M., SISON T.T., ZHANG S., BURG E., YUAN J.X.-J. Diversity of voltage-dependent K+ channels in human pulmonary artery smooth muscle cells. Am. J. Physiol. (Lung, Cell, Mol. Physiol.) 2004;287:L226–L238. doi: 10.1152/ajplung.00438.2003. [DOI] [PubMed] [Google Scholar]
- ROMERO M., REBOREDA A., SÁNCHEZ E., LAMAS J.A. Newly developed blockers of the M-current do not reduce spike frequency adaptation in cultured mouse sympathetic neurons. Eur. J. Neurosci. 2004;19:2693–2702. doi: 10.1111/j.1460-9568.2004.03363.x. [DOI] [PubMed] [Google Scholar]
- RENNIE K.J., WENG T., CORREIA M.J. Effects of KCNQ channel blockers on K+ currents in vestibular hair cells. Am. J. Physiol. (Cell Physiol.) 2001;280:C473–C480. doi: 10.1152/ajpcell.2001.280.3.C473. [DOI] [PubMed] [Google Scholar]
- ROBBINS J. KCNQ potassium channels: physiology, pathophysiology and pharmacology. Pharmacol. Ther. 2001;90:1–19. doi: 10.1016/s0163-7258(01)00116-4. [DOI] [PubMed] [Google Scholar]
- SALEH S.N., GREENWOOD I.A. Activation of chloride currents in murine portal vein smooth muscle cells by membrane depolarisation involves intracellular calcium release. Am. J. Physiol. (Cell Physiol.) 2005;288:C122–C131. doi: 10.1152/ajpcell.00384.2004. [DOI] [PubMed] [Google Scholar]
- SANGUINETTI M.C., CURRAN M.E., ZOU A., SHEN J., SPECTOR P.S., ATKINSON D.L., KEATING M.T. Coassembly of KvLQT1 and minK (IsK) proteins to form the cardiac IKs potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- SCHROEDER B.C., WALDEGGER S., FEHR S., BLEICH M., WARTH R., GREGER R., JENTSCH T.J. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature. 2000;403:195–199. doi: 10.1038/35003200. [DOI] [PubMed] [Google Scholar]
- SEEBOHM G., SCHERER C.R., BUSCH A.E., LERCHE C. Identification of specific pore residues mediating KCNQ1 inactivation. A novel mechanism for long QT syndrome. J. Biol. Chem. 2001;276:13600–13605. doi: 10.1074/jbc.M008373200. [DOI] [PubMed] [Google Scholar]
- SELYANKO A.A., HADLEY J.K., WOOD I.C., ABOGADIE F.C., DELAMS P., BUCKLEY N.J., LONDON B., BROWN D.A. Two types of K+ channel subunit, Erg1 and KCNQ2/3, contribute to the M-like current in a mammalian neuronal cell. J. Neurosci. 1999;19:7742–7756. doi: 10.1523/JNEUROSCI.19-18-07742.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SØGAARD R., LJUNGSTROM T., PEDERSEN K.A., OLESON S.-P., JENSEN B.S. KCNQ4 channels expressed in mammalian cells: functional characteristics and pharmacology. Am. J. Physiol. (Cell Physiol.) 2001;280:C859–C866. doi: 10.1152/ajpcell.2001.280.4.C859. [DOI] [PubMed] [Google Scholar]
- SPENCER N.J., GREENWOOD I.A. Characterization of properties underlying rhythmicity in mouse portal vein. Auton. Neurosci. 2003;104:73–82. doi: 10.1016/S1566-0702(02)00292-8. [DOI] [PubMed] [Google Scholar]
- TINEL N., DIOCHOT S., BORSOTTO M., LAZDUNSKI M., BARHANIN J. KCNE2 confers background current characteristics to the cardiac KCNQ1 potassium channel. EMBO J. 2000;19:6326–6330. doi: 10.1093/emboj/19.23.6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRISTANI-FIROUZI M., SANGUINETTI M.C. Voltage-dependent inactivation of the human K+ channel KvLQT1 is eliminated by association with minimal K+ channel (minK) subunits. J. Physiol. 1998;510:37–45. doi: 10.1111/j.1469-7793.1998.037bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG H.-S., BROWN B.S., MCKINNON D., COHEN I.R. Molecular basis for differential sensitivity of KCNQ and IKs channels to the cognitive enhancer XE991. Mol. Pharmacol. 2000;57:1218–1223. [PubMed] [Google Scholar]
- WANG H.-S., PAN Z., SHI W., BARRY B.S., WYMORE R.S., COHEN I.R., DIXON J.E., MCKINNON D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282:1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- YEUNG S.Y.M., GREENWOOD I.A. Electrophysiological and functional effects of the KCNQ potassium channel blocker XE991 on murine vascular myocytes. Br. J. Pharmacol. 2004;2 (Suppl):009P. doi: 10.1038/sj.bjp.0706342. [DOI] [PMC free article] [PubMed] [Google Scholar]