Abstract
We investigated whether 10 days' in vivo treatment with nitroglycerine (NTG) would inhibit nitric oxide production by the endothelial cells of resistance arteries ex vivo and, if so, what the underlying mechanism might be.
ACh increased the intracellular nitric oxide concentration ([NO]i; estimated using the nitric oxide-sensitive fluorescent dye diaminofluorescein-2) within the endothelial cells of rabbit mesenteric resistance arteries. This effect was significantly smaller in arteries isolated from NTG-treated rabbits than in those from control rabbits. The reduction in endothelial [NO]i in NTG-treated rabbits was prevented when olmesartan (blocker of type 1 angiotensin II receptors (AT1Rs)) was coadministered in vivo with NTG and also when the superoxide scavenger manganese (III) tetrakis-(4-benzoic acid) porphyrin (Mn-TBAP), the protein kinase C (PKC) inhibitor GF109203X or L-arginine (with or without the active form of folate (5-methyltetrahydrofolate)) was incubated with the arteries in vitro.
Endothelial cell superoxide production (estimated by ethidium fluorescence) was greatly increased in arteries from NTG-treated rabbits. This was normalized by in vivo coadministration of olmesartan with NTG and also by in vitro application of Mn-TBAP or GF109203X (but not of 5-methyltetrahydrofolate+L-arginine).
ACh increased the intracellular Ca2+ concentration (estimated using the Ca2+-sensitive dye Fura 2) within endothelial cells, the increase being not significantly different between NTG-treated rabbits and control rabbits.
We conclude that in NTG-treated rabbits, endothelial nitric oxide production in mesenteric resistance arteries is reduced, possibly through a reduction in the bioavailability of L-arginine via an action mediated by superoxide. Activation of the AT1R–PKC pathway may be involved in increasing superoxide production.
Keywords: Nitric oxide, angiotensin II, nitroglycerine, superoxide, endothelial cells
Introduction
Nitroglycerine (NTG) is widely used in the management of such cardiovascular diseases as angina pectoris, acute myocardial infarction and congestive heart failure. Despite their beneficial haemodynamic and anti ischaemic effects, the usefulness of organic nitrates is limited by tolerance, which develops shortly after treatment starts (Parker et al., 1991). More importantly, long-term (e.g. 3–10 days) NTG therapy causes endothelial dysfunction in both the coronary and forearm arterial beds in humans (Caramori et al., 1998; Gori et al., 2001b). In rat and rabbit aortae, the relaxation responses not only to nitrovasodilators but also to endothelium-derived nitric oxide decline after 3 days' in vivo administration of NTG (hereafter referred to as ‘crosstolerance'; Münzel et al., 1995; Laursen et al., 1996; Berkenboom et al., 1999). The mechanisms underlying this crosstolerance may be multifactorial and may involve neurohormonal adjustments (such as activation of the renin–angiotensin–aldosterone axis) as well as changes intrinsic to the vasculature itself (Gori & Parker, 2002a, 2002b).
In conduit arteries, an increase in superoxide production via activation of angiotensin II and/or protein kinase C (PKC) may play a role in the development of the crosstolerance seen in NTG-treated animals (Cai & Harrison, 2000; Münzel et al., 2000; Gori & Parker, 2002a, 2002b). In addition, on the basis of measurements of endothelium-dependent relaxation, nitrite- and/or nitrate production and cGMP-production, it has been suggested that nitric oxide production in vascular endothelial cells may be reduced in both animals and humans treated in vivo with NTG (Gori & Parker, 2002a, 2002b). Superoxide and PKC have been implicated in depletions of the intracellular concentrations of tetrahydrobiopterin and L-arginine in endothelial cells (Graf et al., 2001; Verhaar et al., 2002; Krotova et al., 2003; Alp & Channon, 2004). This may be important since a deficiency of the substrate L-arginine or of the cofactor tetrahydrobiopterin not only reduces nitric oxide production by endothelial nitric oxide synthase (eNOS) but also increases superoxide production via ‘eNOS uncoupling' (Vásquez-Vivar et al., 1998; Gori & Parker, 2002a, 2002b; Kalinowski & Malinski, 2004). In patients with familial hypercholesterolaemia, 5-methyltetrahydrofolate (5-MTHF; an active form of folate) has been reported to restore in vivo endothelial function in forearm veins (in which ‘eNOS uncoupling' has been suggested to develop) (Verhaar et al., 1998), although conflicting evidence has also been published (Woodman et al., 2004). Taken together, these findings suggest that superoxide may inhibit endothelial nitric oxide production through ‘eNOS uncoupling' in large arteries and veins. However, to judge from the available evidence this may not be the full story since superoxide decreases both the bioavailability and effect of nitric oxide (Münzel et al., 2000; Gori & Parker, 2002a, 2002b). Furthermore, it remains unclear whether ‘eNOS uncoupling' develops in resistance arteries, as well as in conduit arteries and veins, following long-term in vivo administration of NTG since the characteristic features of crosstolerance have been suggested to differ among vessel types (Zelis & Mason, 1975; Bassenge & Stewart, 1986; Stewart et al., 1987; Münzel et al., 1996).
A number of methods have been developed for the direct measurement of the intracellular concentration of nitric oxide ([NO]i) within endothelial cells (Christodoulou et al., 1996; Nagano, 1999). However, the detection of nitric oxide ex vivo is hampered by its low production and rapid decomposition in vascular preparations. Although methods for the bioimaging of nitric oxide using electronic paramagnetic resonance and chemiluminescence assays have been developed, they are limited by serious technical drawbacks or low spatial resolution (Leone et al., 1996, Yoshimura et al., 1996). In recent years, the nitric oxide-sensitive fluorescence dye diaminofluorescein-2 (DAF-2) has been employed for the direct estimation of [NO]i (Kojima et al., 1998; Nakatsubo et al., 1998; Dedkova & Blatter, 2002; Murata et al., 2002; Pittner et al., 2003). We recently developed the ability to measure [NO]i by the use of DAF-2 in the endothelial cells of rabbit mesenteric resistance arteries ex vivo.
To clarify whether under ex vivo conditions, endothelial nitric oxide production in resistance arteries is reduced following long-term (10 days) in vivo treatment with NTG, we first observed the increase in endothelial [NO]i (estimated by DAF-2 fluorescence) induced by ACh in mesenteric resistance arteries isolated from NTG-untreated (control) and -treated rabbits. We next studied the effect of in vivo coadministration of the type-1 angiotensin II receptor (AT1R) blocker olmesartan (Mizuno et al., 1995) with the NTG on the ACh-induced increase in endothelial [NO]i. We also observed the in vitro effects of the following agents on the ACh-induced increase in endothelial [NO]i: the superoxide scavenger manganese (III) tetrakis-(4-benzoic acid) porphyrin (Mn-TBAP) (Quijano et al., 2001), the PKC inhibitor GF109203X (Toullec et al., 1991; Nakano et al., 2004), the nitric oxide-synthase substrate L-arginine (either alone or together with 5-MTHF) and the tetrahydrobiopterin precursor sepiapterin (Shimizu et al., 1999).
Methods
Animals
All experiments performed in this study conformed to Guidelines on the Conduct of Animal Experiments issued by the Graduate School of Medical Sciences in Nagoya City University and were approved by the Committee on the Ethics of Animal Experiments in that institution. Male Japan White albino rabbits (supplied by Kitayama Labes, Ina, Japan), weighing 2.5–3.0 kg, were treated by applying transdermal NTG patches (Nitroderm TTS, Novartis Pharma, Tokyo, Japan) to a shaved dorsal thoracic area of the body. Such patches were present continuously for a period of 10 days (each patch being replaced daily with a new one) (‘NTG-treated rabbits'). The theoretical delivery of NTG was 5 mg 24 h−1. Using this protocol, we previously demonstrated the presence of ‘nitrate-tolerance' by showing that the relaxing response to NTG was significantly reduced in the smooth muscle of mesenteric resistance arteries taken from such NTG-treated rabbits (Nakano et al., 2004). In some of the present NTG-treated rabbits, olmesartan medoxomil (referred to hereafter as olmesartan; 1 mg kg−1) suspended in 0.15 % carboxymethylcellulose solution was administered orally once a day for the same 10-day period (‘NTG+olmesartan-treated rabbits'). Male rabbits of a similar body weight served as controls (‘control rabbits').
Blood pressure measurement
In some rabbits, mean arterial blood pressure was measured via an ear-artery catheter under light anaesthesia (pentobarbitone sodium 20 mg kg−1 given intravenously (i.v.)). The pressure was continuously recorded for over 15 min and the mean pressure was averaged over the last 5-min period.
Tissue preparation
Rabbits were anaesthetized by injection of pentobarbitone sodium (40 mg kg−1 given i.v.), then killed by exsanguination. The third and fourth branches of the mesenteric artery distributing to the region of the ileum (diameter, approximately 120–150 μm) were immediately excised and placed in Krebs solution, then cleaned by removal of connective tissue. After each artery had been cut along its long axis using a small scissors, circularly cut strips were carefully prepared so as not to damage the endothelium. In some experiments, the endothelium was carefully removed by gentle rubbing of the intimal surface of the vessel using small pieces of razor blade, as previously described (Nakano et al., 2004).
Fluorescence detection
Fluorescence signals were detected using a CCD camera (C6790; Hamamatsu Photonics, Hamamatsu, Japan) fitted to an inverted fluorescence microscope (ECLIPSE TE300; Nikon, Tokyo, Japan), which allowed superfusion with the experimental solution. The microscope was equipped with objective lenses of a high numerical aperture (N.A.) (for × 20 objective, N.A.=0.75, Nikon; for × 40 oil immersion objective, N.A.=1.3, Nikon) and with filters appropriate for fluorescence microscopy.
An endothelium-intact strip was placed in a chamber of 1 ml volume with the luminal side down. Then, each end of the preparation was fixed using a small tungsten wire (diameter 0.02 mm), great care being taken to keep the endothelium intact. After the strip had been loaded with an appropriate fluorescent dye, the chamber was transferred to the fluorescence microscope. The focus was adjusted to reveal individual endothelial cells and the experiment was started after a 30-min perfusion with Krebs solution (at a flow rate of about 1 ml min−1) under dark conditions. The images were captured and analysed using commercial software (AquaCosmos; Hamamatsu Photonics). These experiments were performed in such a way that control and test images were captured under the same conditions.
Measurement of [NO]i
The [NO]i within endothelial cells was estimated from the increase in the fluorescence intensity of the nitric oxide-sensitive dye DAF-2 (Kojima et al., 1998; Nakatsubo et al., 1998). Endothelium-intact strips were exposed to membrane-permeable DAF-2 diacetate (10 μM) for 40 min at 37°C in Krebs solution, then washed with Krebs solution for 30 min. Next, ACh (3 μM) was applied for 15 min to the strips. DAF-2 was excited at 490 nm (half-width, 20 nm) at 60-s intervals and the DAF-2 fluorescence intensity within endothelial cells at any given time after the application of ACh (F) was normalized with respect to the fluorescence intensity just before the application of ACh (F0) in the same experiment. Thus, changes in [NO]i are expressed as F/F0. The mean fluorescence intensities obtained from five endothelial cells in each strip were averaged and this value (one value per strip) was used for the later analysis. All experiments were performed at 37°C.
The superoxide scavenger Mn-TBAP (40 μM), the PKC inhibitor GF109203X (3 μM), the eNOS substrate L-arginine (1 mM; either alone or together with the active form of folate, 5-MTHF (100 μM)), the tetrahydrobiopterin precursor sepiapterin (100 μM) or the nitric oxide-synthase inhibitor Nω-nitro-L-arginine (L-NNA, 0.1 mM) was applied to strips before and during the loading of DAF-2 (3 h application in total). ACh (3 μM) was then applied in the absence of the above agents.
Measurement of intracellular concentration of Ca2+ ([Ca2+]i)
The [Ca2+]i was estimated using the ratiometric fluorescence Ca2+-indicator Fura 2. Endothelium-intact strips were loaded with 5 μM Fura 2 acetoxymethyl ester (Fura 2-AM) in Krebs solution containing 0.001% Pluronic F-127 for 3 h at room temperature, then washed with Krebs solution for 30 min. Next, ACh (3 μM) was applied for 15 min to the strips. Fura 2 was excited by dual wavelengths (340 nm (F340) and 380 nm (F380)) for 182 ms and collected through a 510 nm emission filter (half-width, 20 nm) at 15-s intervals. Changes in [Ca2+]i were expressed as the change in the fluorescence ratio F340/F380. The mean fluorescence intensities obtained from five endothelial cells in each strip were averaged and this value (one value per strip) was used for the later analysis. All the experiments were performed at 37°C.
Superoxide production
The oxidative fluorescent dye dihydroethidium was used to detect superoxide production. Dihydroethidium, upon oxidation by intracellular superoxide anions, is converted to ethidium. This binds irreversibly to DNA, producing a bright red fluorescence. Segments (2.5 mm long) of endothelium-intact mesenteric resistance arteries were incubated with Krebs solution at 37°C for 3 h, then frozen in O.C.T. Compound (Tissue Tek; SAKURA Finetechnical, Tokyo, Japan). When the effects of Mn-TBAP (40 μM), GF109203X (3 μM) or L-arginine (1 mM)+5-MTHF (100 μM) were to be examined, strips were incubated with the appropriate agent(s) for 3 h at 37°C before being frozen.
Transverse sections (10 μm thickness) were prepared on a cryostat (Microtome Cryostat HM 550; MICROM International GmbH, Walldorf, Germany) and placed on MAS-coated glass slides (Matsunami Glass, Kishiwada, Japan). They were then incubated in a light-protected chamber at 37°C for 30 min with 2 μM dihydroethidium. Images were obtained using a confocal laser-scanning microscope system (LSM5 PASCAL; Carl Zeiss, Jena, Germany). The excitation wavelength was 488 nm and emission fluorescence was detected through a 585 nm long-pass filter. Identical laser settings were used for the acquisition of images from different groups of rabbits. Fluorescence images were acquired as 12-bit files for subsequent analysis using commercial software supplied for the LSM5 PASCAL system (Carl Zeiss).
The changes in superoxide production in endothelium-intact strips of rabbit mesenteric resistance arteries (length 10–12 mm, width 0.5–0.6 mm) were also examined by using the superoxide-sensitive chemiluminescence dye 8-amino-5-chloro-7-phenylpyridol[3,4-d]pyridazine-1,4-(2H,3H)dione sodium salt (L-012, 100 μM; Daiber et al., 2004) and a luminometer (Multi-biolumat LB 9505C; Berthold, Bad Wildbad, Germany).
Solutions
The ionic composition of the Krebs solution used was as follows (mM): 137.4 Na+, 5.9 K+, 1.2 Mg2+, 2.6 Ca2+, 15.5 HCO3−, 1.2 H2PO4−, 134 Cl− and 11.5 glucose. Nominal Ca2+-free solution was prepared by replacing calcium chloride with magnesium chloride isosmotically (no EGTA added). The solutions were bubbled with 95% oxygen and 5% carbon dioxide.
Drugs
The drugs used in the current experiments were as follows: 5-MTHF and sepiapterin (Sigma Chemical Co., St Louis, MO, U.S.A.), Mn-TBAP (Alexis Biochemicals, San Diego, CA, U.S.A.), GF109203X (Tocris Cookson, Avonmouth, U.K.), dihydroethidium (Molecular Probes, Eugene, OR, U.S.A.), L-012 and L-arginine (Wako Pure Chemical, Tokyo, Japan), L-NNA (Peptide Institute Inc., Osaka, Japan), ACh-HCl (Daiichi Pharmaceutical, Tokyo, Japan), DAF-2 diacetate (Daiichi Pure Chemicals, Tokyo, Japan) and Fura 2-AM (Dojindo, Kumamoto, Japan). Olmesartan medoxomil (olmesartan) was kindly provided by Sankyo Pharmaceutical Co. (Tokyo, Japan).
GF109203X (10 mM) and sepiapterin (100 mM) were each dissolved in DMSO to make stock solutions, while Mn-TBAP was dissolved in ethanol (as a 50 mM stock solution). All other drugs were dissolved in ultra-pure Milli-Q water (Japan Millipore Corp., Tokyo, Japan). The stock solutions were stored at −80°C and diluted in Krebs solution to the required final concentrations immediately before use.
Statistical analysis
All results are expressed as the mean±s.e.m. The n values represent the number of rabbits used. A two-way repeated-measures ANOVA (followed by Scheffé's F-test for post hoc analysis) or a Student's unpaired t-test with an F-test were used for statistical analysis. The level of significance was set at P<0.05.
Results
Blood pressure
The mean arterial blood pressure in NTG-treated rabbits (86.3±2.5 mmHg, n=3) was not significantly different from that in NTG-untreated control rabbits (85.3±1.9 mmHg, n=3; P>0.1). The blood pressure tended to be lower, although not significantly, in NTG+olmesartan-treated rabbits (80.7±3.2 mmHg, n=3; P>0.1 versus control rabbits).
Nitric oxide production by endothelial cells
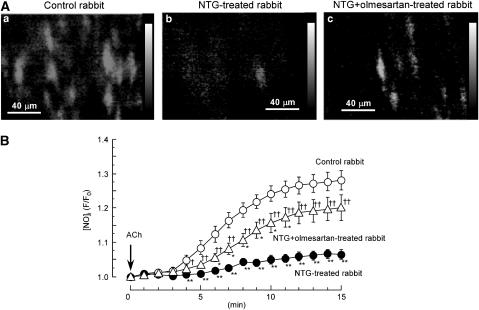
Using an objective lens with a relatively high numerical aperture, the focus was adjusted to reveal the intimal surface of a mesenteric resistance artery. Under our experimental conditions, DAF-2 fluorescence was only visible within the endothelial cells. ACh (3 μM) progressively increased the DAF-2 fluorescence ratio until about 15 min after its application (Figure 1A and B). The nitric oxide synthase inhibitor L-NNA (0.1 mM) abolished the ACh-induced increase in the DAF-2 fluorescence ratio in control rabbits (the ratio=1.01±0.03 at 15 min after ACh application, n=7; Figure 2a). ACh did not modify the DAF-2 fluorescence ratio in endothelium-denuded strips or in endothelium-intact strips in nominal Ca2+-free solution (data not shown). The above ACh-induced increase in the DAF-2 fluorescence ratio was significantly smaller in arteries from NTG-treated rabbits (n=17) than in those from control rabbits (n=13) (P<0.001, two-way repeated-measures ANOVA; Figure 1Ab and 1B). The in vivo administration of the AT1R blocker olmesartan with the NTG largely prevented the above effect of NTG (n=10; P<0.05 versus control rabbits and P<0.01 versus NTG-treated rabbits, two-way repeated-measures ANOVA; Figure 1Ac and 1B). By contrast, in control rabbits, the ACh-induced increase in nitric oxide production was not significantly modified by the in vivo administration of olmesartan for 10 days (the ratio=1.25±0.05 at 15 min after ACh application, n=3; P>0.1 versus olmesartan-untreated control rabbits).
Figure 1.
ACh-induced increase in [NO]i in endothelial cells of rabbit mesenteric resistance arteries. (A) Fluorescence ratio images of the nitric oxide-sensitive dye DAF-2 (taken at 15 min after application of 3 μM ACh) in endothelial cells. When used, olmesartan (blocker of type 1 angiotensin II receptors (AT1Rs)) was coadministered in vivo with NTG. Fluorescence ratio was taken as the fluorescence intensity at 15 min after ACh application (F) divided by the fluorescence intensity just before ACh application (F0) in each pixel. (B) Effect of ACh on DAF-2 fluorescence ratio as a function of time. [NO]i is expressed as the ratio of F (fluorescence intensity at a given time after ACh application) to F0 (just before ACh application). Data are shown as mean±s.e.m. **P<0.01 versus ‘Control rabbit'; †P<0.05, ††P<0.01 versus ‘NTG-treated rabbit' (two-way repeated-measures ANOVA followed by Scheffé's F-test for post hoc analysis).
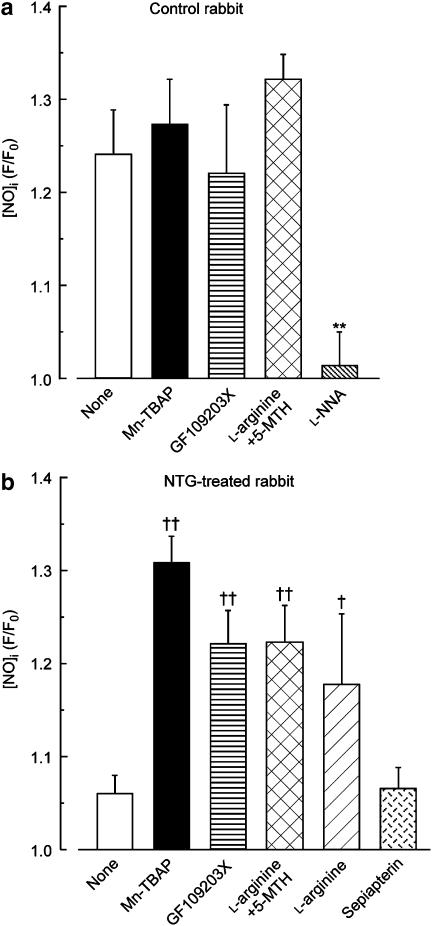
Figure 2.
Effects of in vitro applications of L-NNA, Mn-TBAP, GF109203X, L-arginine (with or without 5-MTHF) and sepiapterin on ACh-induced increase in [NO]i in endothelial cells of rabbit mesenteric resistance arteries. (a) Control rabbits. (b) NTG-treated rabbits. Increase in [NO]i is expressed as ratio of F (fluorescence intensity at 15 min after ACh application) to F0 (just before ACh application). Data are shown as mean±s.e.m. **P<0.01 versus ‘None' in control rabbits; †P<0.05, ††P<0.01 versus ‘None' in NTG-treated rabbits (Student's unpaired t-test).
Effects of Mn-TBAP, GF109203X, L-arginine (with or without 5-MTHF) and sepiapterin on nitric oxide production by the endothelial cells
In arteries from control rabbits, various treatments (the superoxide scavenger Mn-TBAP (40 μM), the PKC inhibitor GF109203X (3 μM) and the nitric oxide-synthase substrate L-arginine (1 mM) plus the active form of folate 5-MTHF (100 μM)) all failed significantly to modify nitric oxide production by endothelial cells at 15 min after an application of ACh (Figure 2a). By contrast, the reduced ACh-induced nitric oxide production seen in arteries from NTG-treated rabbits was significantly enhanced by all three of these treatments, towards the level seen in arteries from control rabbits (in each case, n=7; P<0.01 versus ‘None' in NTG-treated rabbits; Figure 2b). Similarly, the reduced ACh-induced nitric oxide production seen in NTG-treated rabbits was restored by an application of L-arginine (1 mM) alone (n=4; P<0.05 versus ‘None' in NTG-treated rabbits) but not by sepiapterin (100 μM; n=6; P>0.1; Figure 2b). In magnitude, the enhancement induced by L-arginine alone was similar to those induced by Mn-TBAP, GF109203X and L-arginine+5-MTHF (P>0.1 in each case; Figure 2b).
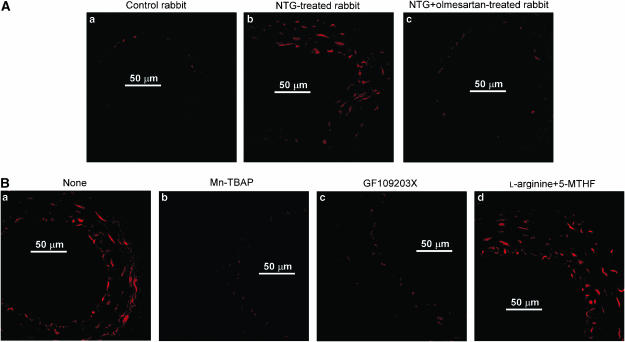
Superoxide production within the vascular wall
In arteries from control rabbits, a faint superoxide production (estimated from ethidium fluorescence) was seen within a few endothelial cells (Figure 3Aa). In arteries from NTG-treated rabbits, superoxide production was very much increased within endothelial cells and was also seen in some smooth muscle cells (Figure 3Ab). It was normalized when olmesartan was coadministered in vivo with the NTG (Figure 3Ac). The increased superoxide production seen within the vascular wall in arteries from NTG-treated rabbits was greatly reduced when strips were coincubated for 3 h with either Mn-TBAP (Figure 3Bb) or GF109203X (Figure 3Bc) in vitro. By contrast, L-arginine+5-MTHF had no such effect (Figure 3Bd).
Figure 3.
Superoxide production within vascular walls of rabbit mesenteric resistance arteries. (A) Superoxide production was estimated from the fluorescence intensity of the superoxide-sensitive dye dihydroethidium. (B) Effects of in vitro applications of Mn-TBAP, GF109203X and L-arginine+5-MTHF on superoxide production in NTG-treated rabbits. For both (A) and (B), similar observations were made in other sections obtained from four preparations from four other animals.
In endothelium-intact strips, the chemiluminescence intensities of L-012 (100 μM, a superoxide-sensitive chemiluminescence dye) were not significantly different among the control rabbits, NTG-treated rabbits and NTG+olmesartan-treated rabbits. In the absence of SOD, the values (counts min−1 mg dry weight−1) were 22,777±1186, 20,707±1402 and 23,730±3843 for control, NTG- and NTG+olmesartan-treated rabbits, respectively (n=3 for each group, P>0.1). An addition of SOD (200 U ml−1) caused total abolition of these signals (282±321, −68±983 and −73±576 counts min−1 mg dry weight−1 in control, NTG- and NTG+olmesartan-treated rabbits, respectively).
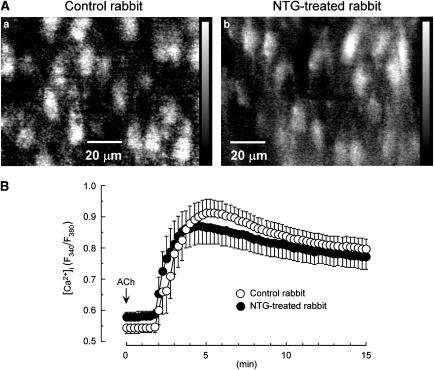
[Ca2+]i increase within endothelial cells
The resting values obtained for the Fura 2 ratio in endothelial cells in rabbit mesenteric arteries were not significantly different between control rabbits (0.54±0.02, n=9) and NTG-treated rabbits (0.58±0.02, n=6, P>0.1). ACh (3 μM) induced a rapid transient increase in this ratio, followed by a tonic increase (with a slight decline as a function of time until 15 min) in arteries from both control rabbits and NTG-treated rabbits (Figure 4A and 4B). This ACh-induced increase was not significantly different between the two groups (n=4 for each group; P>0.1, two-way repeated-measures ANOVA).
Figure 4.
ACh-induced increase in [Ca2+]i in endothelial cells of rabbit mesenteric resistance arteries. (A) Fluorescence ratio images of the Ca2+-sensitive dye Fura 2 at 15 min after application of 3 μM ACh. (B) ACh-induced increase in [Ca2+]i as a function of time. [Ca2+]i is expressed as the Fura 2 ratio (F340/F380). Data are shown as mean±s.e.m.
Discussion
In the present study, we found that under ex vivo conditions, the ACh-induced increase in [NO]i in endothelial cells was significantly smaller in mesenteric resistance arteries from NTG-treated rabbits than in those from NTG-untreated control rabbits. We also found (a) that the intensity of the fluorescence due to the oxidative fluorescence dye dihydroethidium within vascular cells (including endothelial cells) was greatly increased in the NTG-treated group and (b) that this was normalized by the in vitro application of the cell-permeable superoxide scavenger Mn-TBAP. Although the assessment of superoxide production in the vascular wall using dihydroethidium is semiquantitative, these results suggest that superoxide production by vascular cells in mesenteric resistance arteries is increased in NTG-treated rabbits. Moreover, Mn-TBAP enhanced the ACh-induced increase in endothelial [NO]i in this resistance artery only in the NTG-treated group. These results indicate that long-term (10 days) in vivo administration of NTG reduces ACh-induced endothelial nitric oxide production by an action that is probably mediated via an increased cellular production of superoxide in rabbit mesenteric resistance arteries, just as it has been postulated to do in conduit arteries (Münzel et al., 1995; 1999; 2000; Kurz et al., 1999).
In the present experiments, the chemiluminescence intensities of L-012 (a superoxide-sensitive chemiluminescence dye) shown by the endothelium-intact strips of rabbit mesenteric resistance arteries were not significantly different among the control rabbits, NTG-treated rabbits and NTG+olmesartan-treated rabbits. Since addition of SOD caused total abolition of these signals, we believe that our system appropriately measured the production of superoxide, or at least that located in the extracellular space. Therefore, it may be that the chemiluminescence-based assay (using L-012) is not sensitive enough to detect changes in the production of superoxide in both the intracellular and extracellular spaces in such small segments of mesenteric resistance arteries from NTG-treated rabbits.
Reduced production of nitric oxide by endothelial cells in NTG-treated rabbits
eNOS contains two functionally distinct domains: an N-terminal oxygenase domain (containing binding sites for haem, tetrahydrobiopterin and L-arginine) and a C-terminal reductase domain (containing binding sites for FAD, FMN and NAD(P)H). These two domains are linked by a calmodulin-binding site. In this site, upon Ca2+ binding, calmodulin increases the rate of electron transfer from NAD(P)H to the reductase-domain flavins and from the reductase domain to the haem centre for oxidation of the cosubstrates O2 and L-arginine, thereby producing nitric oxide and L-citrulline (Palmer et al., 1988; Moncada et al., 1991; Hemmens & Mayer, 1998). The presence of agonists or shear stress enhances the production of nitric oxide through an increase in [Ca2+]i within endothelial cells and subsequently activates the cascade described above to produce nitric oxide. We found that in the endothelial cells of rabbit mesenteric resistance arteries, the increase in [Ca2+]i induced by ACh was not altered in the NTG-treated group (compared with that in the NTG-untreated control group). This indicates that an abnormality in the downstream signals following the increase in [Ca2+]i within the endothelial cell is responsible for the downregulation of nitric oxide production seen in rabbits treated in vivo with NTG.
Superoxide reduces [NO]i through its binding with nitric oxide to form peroxynitrite, which in turn uncouples eNOS through an oxidation of the eNOS cofactor tetrahydrobiopterin (Vásquez-Vivar et al., 1998; Gori & Parker, 2002a). Furthermore, long-term (3 days) in vivo administration of NTG may reduce the cellular concentration of the eNOS substrate L-arginine through an inhibition of its uptake into endothelial cells, as found in cultured bovine aortic endothelial cells (Abou-Mohamed et al., 2000). In conditions in which there is a deficiency of tetrahydrobiopterin or L-arginine, eNOS cannot catalyse the five-electron oxidation of L-arginine to nitric oxide, resulting in a decreased production of nitric oxide and, instead, an increased production of superoxide in high [Ca2+]i conditions (Wever et al., 1997; Raman et al., 1998; Vásquez-Vivar et al., 1998). It has been suggested that the active form of folate, 5-MTHF, increases nitric oxide production through an enhanced binding of tetrahydrobiopterin to eNOS and/or an increased tetrahydrobiopterin availability (Stroes et al., 2000; Heller et al., 2001; Loscalzo, 2001; Verhaar et al., 2002). Moreover, it has been found that (i) 5-MTHF supplementation prevents the endothelial dysfunction induced by the chronic administration of NTG in healthy subjects (Gori et al., 2001a) and (ii) L-arginine administration improves tolerance to NTG in patients with angina (Parker et al., 2002). These pieces of evidence suggest that in the setting of nitrate tolerance, a cellular deficiency not only of tetrahydrobiopterin but also of L-arginine may be involved in the reduced nitric oxide production that exists in human vascular endothelial cells. In the present experiments, we found that in mesenteric resistance arteries from NTG-treated rabbits, the ACh-induced [NO]i increase in endothelial cells was enhanced by an in vitro application of L-arginine alone to the same extent as by coapplication of L-arginine and 5-MTHF. Furthermore, the tetrahydrobiopterin precursor sepiapterin alone had no effect on the ACh-induced [NO]i increase in NTG-treated rabbits. These results suggest that a reduction in the availability of intracellular L-arginine (rather than of tetrahydrobiopterin) may be causally involved in the reduced ACh-induced [NO]i increase seen in the endothelial cells of mesenteric resistance arteries from NTG-treated rabbits.
Role of AT1R in superoxide production
In animals and humans, in vivo administration of NTG activates the renin–angiotensin–aldosterone axis via activation of a neurohormonal counter-regulatory mechanism (Münzel et al., 1996; Gori & Parker, 2002a, 2002b). Angiotensin II binds to AT1R and enhances the activity and/or expression of membrane-bound forms of NAD(P)H oxidases, thus increasing superoxide production by vascular cells (Touyz & Schiffrin, 2000; Mollnau et al., 2002; Kalinowski & Malinski, 2004). In the present experiments, in vivo coadministration of the AT1R blocker olmesartan with NTG normalized the increased superoxide production by the endothelial cells of mesenteric resistance arteries that was seen in the NTG-treated group. However, it has been reported that another AT1R blocker, losartan, does not prevent the tolerance to NTG shown by blood pressure and forearm-venous-volume responses in humans (Milone et al., 1999). Furthermore, it has been noted that losartan needs to be given at a high dose (10–25 mg kg−1) to be very effective at preventing the nitrate tolerance seen in the aorta of NTG-treated rabbits (Kurz et al., 1999), and high doses are also needed in the case of angiotensin-converting-enzyme (ACE) inhibitors (Muiesan et al., 1993; Münzel et al., 1996). Taken together, these results suggest that to prevent nitrate tolerance by modulating the renin–angiotensin system, a powerful inhibition needs to be produced (e.g. by high-dose ACE inhibition or high-dose AT1R blockade).
It has been suggested that PKC may, to some extent, mediate the above action of angiotensin II (Münzel et al., 1995; Harrison, 1997; Heitzer et al., 1999). In the present study, we found that in vitro application of the PKC inhibitor GF109203X normalized superoxide production by endothelial cells in arteries from NTG-treated rabbits, suggesting that long-term in vivo administration of NTG enhances superoxide production via an activation of AT1R–PKC pathways within the endothelial cells of rabbit mesenteric resistance arteries. It was recently found that both NTG (Abou-Mohamed et al., 2004) and the nitric oxide donor S-nitroso-N-acetyl-DL-penicillamine (Balafanova et al., 2002) activate PKC in endothelial cells via a mechanism apparently unrelated to the renin–angiotensin system. Furthermore, the transport of L-arginine into endothelial cells is downregulated by activation of PKC (Graf et al., 2001; Krotova et al., 2003). Taken together, these results suggest that activation of PKC may play a pivotal role in the downregulation of endothelial nitric oxide production seen in the setting of nitrate tolerance.
It is known that NTG tolerance affects the relaxation responses to other nitrovasodilators, as well as to endothelium-derived nitric oxide (‘crosstolerance'; Gori & Parker, 2002a, 2002b). Several possible mechanisms need to be considered for this crosstolerance, such as (i) a decrease in the bioavailability of nitric oxide (White et al., 1997), (ii) a desensitization of soluble guanylyl cyclase (Molina et al., 1987; Mollnau et al., 2002) and/or (iii) a reduction in cGMP-mediated relaxation (Soff et al., 1997; Nakano et al., 2004). In the present experiments, we found that ACh-induced nitric oxide production is reduced in the endothelial cells of mesenteric resistance arteries from rabbits treated with NTG. In the setting of NTG-tolerance, however, there seem to be differential effects among various types of blood vessels and species in the responsiveness to NTG (Zelis & Mason, 1975; Stewart et al., 1986; 1987; Münzel et al., 1996). Thus, we must stress the need for caution before any attempt is made to extrapolate the present results to other vascular beds and/or different species, in which additional mechanisms not addressed by the present experiments may help to underpin nitrate tolerance.
In conclusion, 10 days in vivo administration of NTG leads to a reduction in nitric oxide production by the endothelial cells of the rabbit mesenteric resistance artery. It is suggested that a decrease in the bioavailability of L-arginine resulting from an increased concentration of superoxide (through an activation of AT1R–PKC pathways) within the endothelial cells may be the underlying mechanism.
Abbreviations
- AT1R
type-1 angiotensin II receptor
- [Ca2+]i
intracellular concentration of Ca2+
- DAF-2
diaminofluorescein-2
- L-012
8-amino-5-chloro-7-phenylpyridol[3,4-d]pyridazine-1,4-(2H,3H)dione sodium salt
- L-NNA
Nω-nitro-L-arginine
- Mn-TBAP
manganese (III) tetrakis-(4-benzoic acid) porphyrin
- 5-MTHF
5-methyltetrahydrofolate
- [NO]i
intracellular concentration of nitric oxide
- eNOS
endothelial nitric oxide synthase
- NTG
nitroglycerine
References
- ABOU-MOHAMED G., JOHNSON J.A., JIN L., EL-REMESSY A.B., DO K., KAESEMEYER W.H., CALDWELL R.B., CALDWELL R.W. Roles of superoxide, peroxynitrite, and protein kinase C in the development of tolerance to nitroglycerin. J. Pharmacol. Exp. Ther. 2004;308:289–299. doi: 10.1124/jpet.103.056119. [DOI] [PubMed] [Google Scholar]
- ABOU-MOHAMED G., KAESEMEYER W.H., CALDWELL R.B., CALDWELL R.W. Role of L-arginine in the vascular actions and development of tolerance to nitroglycerin. Br. J. Pharmacol. 2000;130:211–218. doi: 10.1038/sj.bjp.0703293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALP N.J., CHANNON K.M. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler. Thromb. Vasc. Biol. 2004;24:413–420. doi: 10.1161/01.ATV.0000110785.96039.f6. [DOI] [PubMed] [Google Scholar]
- BALAFANOVA Z., BOLLI R., ZHANG J., ZHENG Y., PASS JM., BHATNAGAR A., TANG X.L., WANG O., CARDWELL E., PING P. Nitric oxide (NO) induces nitration of protein kinase Cɛ (PKCɛ), facilitating PKCɛ translocation via enhanced PKCɛ–RACK2 interactions: a novel mechanism of NO-triggered activation of PKCɛ. J. Biol. Chem. 2002;277:15021–15027. doi: 10.1074/jbc.M112451200. [DOI] [PubMed] [Google Scholar]
- BASSENGE E., STEWART D.J. Effects of nitrates in various vascular sections and regions. Z. Kardiol. 1986;75 (Suppl. 3):1–7. [PubMed] [Google Scholar]
- BERKENBOOM G., FONTAINE D., UNGER P., BALDASSARRE S., PREUMONT N., FONTAINE J. Absence of nitrate tolerance after long-term treatment with ramipril: an endothelium-dependent mechanism. J. Cardiovasc. Pharmacol. 1999;34:547–553. doi: 10.1097/00005344-199910000-00011. [DOI] [PubMed] [Google Scholar]
- CAI H., HARRISON D.G. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ. Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- CARAMORI P.R., ADELMAN A.G., AZEVEDO E.R., NEWTON G.E., PARKER A.B., PARKER J.D. Therapy with nitroglycerin increases coronary vasoconstriction in response to acetylcholine. J. Am. Coll. Cardiol. 1998;32:1969–1974. doi: 10.1016/s0735-1097(98)00456-2. [DOI] [PubMed] [Google Scholar]
- CHRISTODOULOU D., KUDO S., COOK J.A., KRISHNA M.C., MILES A., GRISHAM M.B., MURUGESAN M., FORD P.C., WINK D.A. Electrochemical methods for detection of nitric oxide. Methods Enzymol. 1996;268:69–83. doi: 10.1016/s0076-6879(96)68010-0. [DOI] [PubMed] [Google Scholar]
- DAIBER A., AUGUST M., BALDUS S., WENDT M., OELZE M., SYDOW K., KLESCHYOV A.L., MÜNZEL T. Measurement of NAD(P)H oxidase-derived superoxide with the luminol analogue L-012. Free Radic. Biol. Med. 2004;36:101–111. doi: 10.1016/j.freeradbiomed.2003.10.012. [DOI] [PubMed] [Google Scholar]
- DEDKOVA E.N., BLATTER L.A. Nitric oxide inhibits capacitative Ca2+ entry and enhances endoplasmic reticulum Ca2+ uptake in bovine vascular endothelial cells. J. Physiol. 2002;539:77–91. doi: 10.1113/jphysiol.2001.013258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORI T., BURSTEIN J.M., AHMED S., MINER S.E.S., AL-HESAYEN A., KELLY S., PARKER J.D. Folic acid prevents nitroglycerin-induced nitric oxide synthase dysfunction and nitrate tolerance: a human in vivo study. Circulation. 2001a;104:1119–1123. doi: 10.1161/hc3501.095358. [DOI] [PubMed] [Google Scholar]
- GORI T., MAK S.S., KELLY S., PARKER J.D. Evidence supporting abnormalities in nitric oxide synthase function induced by nitroglycerin in humans. J. Am. Coll. Cardiol. 2001b;38:1096–1101. doi: 10.1016/s0735-1097(01)01510-8. [DOI] [PubMed] [Google Scholar]
- GORI T., PARKER J.D. The puzzle of nitrate tolerance: pieces smaller than we thought. Circulation. 2002a;106:2404–2408. doi: 10.1161/01.cir.0000036742.52907.91. [DOI] [PubMed] [Google Scholar]
- GORI T., PARKER J.D. Nitrate tolerance: a unifying hypothesis. Circulation. 2002b;106:2510–2513. doi: 10.1161/01.cir.0000036743.07406.53. [DOI] [PubMed] [Google Scholar]
- GRAF P., FORSTERMANN U., CLOSS E.I. The transport activity of the human cationic amino acid transporter hCAT-1 is downregulated by activation of protein kinase C. Br. J. Pharmacol. 2001;32:1193–1200. doi: 10.1038/sj.bjp.0703921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRISON D.G. Endothelial function and oxidant stress. Clin. Cardiol. 1997;20:II11–II17. [PubMed] [Google Scholar]
- HEITZER T., WENZEL U., HINK U., KROLLNER D., SKATCHKOV M., STAHL R.A., MACHARZINA R., BRASEN J.H., MEINERTZ T., MÜNZEL T. Increased NAD(P)H oxidase-mediated superoxide production in renovascular hypertension: evidence for an involvement of protein kinase C. Kidney Int. 1999;55:252–260. doi: 10.1046/j.1523-1755.1999.00229.x. [DOI] [PubMed] [Google Scholar]
- HELLER R., UNBEHAUM A., SCHELLENBERG B., MAYER B., WERNER-FELMAYER G., WERNER E.R. L-ascorbic acid potentiates endothelial nitric oxide synthase via chemical stabilization of tetrahydrobiopterin. J. Biol. Chem. 2001;276:40–47. doi: 10.1074/jbc.M004392200. [DOI] [PubMed] [Google Scholar]
- HEMMENS B., MAYER B. Enzymology of nitric oxide synthases. Methods Mol. Biol. 1998;100:1–32. doi: 10.1385/1-59259-749-1:1. [DOI] [PubMed] [Google Scholar]
- KALINOWSKI L., MALINSKI T. Endothelial NADH/NADPH-dependent enzymatic sources of superoxide production: relationship to endothelial dysfunction. Acta Biochim. Pol. 2004;51:459–469. [PubMed] [Google Scholar]
- KOJIMA H., NAKATSUBO N., KIKUCHI K., KAWAHARA S., KIRINO Y., NAGOSHI H., HIRATA Y., NAGANO T. Detection and imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal. Chem. 1998;70:2446–2453. doi: 10.1021/ac9801723. [DOI] [PubMed] [Google Scholar]
- KROTOVA K.Y., ZHARIKOV S.I., BLOCK E.R. Classical isoforms of PKC as regulators of CAT-1 transporter activity in pulmonary artery endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;284:L1037–L1044. doi: 10.1152/ajplung.00308.2002. [DOI] [PubMed] [Google Scholar]
- KURZ S., HINK U., NICKENIG G., BORTHAYRE A.B., HARRISON D.G., MÜNZEL T. Evidence for a causal role of the renin–angiotensin system in nitrate tolerance. Circulation. 1999;99:3181–3187. doi: 10.1161/01.cir.99.24.3181. [DOI] [PubMed] [Google Scholar]
- LAURSEN J.B., MULSCH A., BOESGAARD S., MORDVINTCEV P., TRAUTNER S., GRUHN N., NIELSEN-KUDSK J.E., BUSSE R., ALDERSHVILE J. In vivo nitrate tolerance is not associated with reduced bioconversion of nitroglycerin to nitric oxide. Circulation. 1996;94:2241–2247. doi: 10.1161/01.cir.94.9.2241. [DOI] [PubMed] [Google Scholar]
- LEONE A.M., FURST V.W., FOXWELL N.A., CELLEK S., MONCADA S. Visualisation of nitric oxide generated by activated murine macrophages. Biochem. Biophys. Res. Commun. 1996;221:37–41. doi: 10.1006/bbrc.1996.0557. [DOI] [PubMed] [Google Scholar]
- LOSCALZO J. Folate and nitrate-induced endothelial dysfunction: a simple treatment for a complex pathobiology. Circulation. 2001;104:1086–1088. [PubMed] [Google Scholar]
- MILONE S.D., AZEVEDO E.R., FORSTER C., PARKER J.D. The angiotensin II-receptor antagonist losartan does not prevent hemodynamic or vascular tolerance to nitroglycerin. J. Cardiovasc. Pharmacol. 1999;34:645–650. doi: 10.1097/00005344-199911000-00004. [DOI] [PubMed] [Google Scholar]
- MIZUNO M., SADA T., IKEDA M., FUKUDA N., MIYAMOTO M., YANAGISAWA H., KOIKE H. Pharmacology of CS-866, a novel nonpeptide angiotensin II receptor antagonist. Eur. J. Pharmacol. 1995;285:181–188. doi: 10.1016/0014-2999(95)00401-6. [DOI] [PubMed] [Google Scholar]
- MOLINA C.R., ANDRESEN J.W., RAPOPORT R.M., WALDMAN S., MURAD F. Effect of in vivo nitroglycerin therapy on endothelium-dependent and independent vascular relaxation and cyclic GMP accumulation in rat aorta. J. Cardiovasc. Pharmacol. 1987;10:371–378. doi: 10.1097/00005344-198710000-00001. [DOI] [PubMed] [Google Scholar]
- MOLLNAU H., WENDT M., SZOCS K., LASSEGUE B., SCHULZ E., OELZE M., LI H., BODENSCHATZ M., AUGUST M., KLESCHYOV A.L., TSILIMINGAS N., WALTER U., FORSTERMANN U., MEINERTZ T., GRIENDLING K., MÜNZEL T. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ. Res. 2002;90:E58–E65. doi: 10.1161/01.res.0000012569.55432.02. [DOI] [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M., HIGGS E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- MUIESAN M.L., BONI E., CASTELLANO M., BESCHI M., CEFIS G., CERRI B., VERDECCHIA P., PORCELLATI C., POLLAVINI G., AGABITI-ROSEI E. Effects of transdermal nitroglycerin in combination with an ACE inhibitor in patients with chronic stable angina pectoris. Eur. Heart J. 1993;14:1701–1708. doi: 10.1093/eurheartj/14.12.1701. [DOI] [PubMed] [Google Scholar]
- MÜNZEL T., HEITZER T., KURZ S., HARRISON D.G., LUHMAN C., PAPE L., OLSCHEWSKI M., JUST H. Dissociation of coronary vascular tolerance and neurohormonal adjustments during long-term nitroglycerin therapy in patients with stable coronary artery disease. J. Am. Coll. Cardiol. 1996;27:297–303. doi: 10.1016/0735-1097(95)00475-0. [DOI] [PubMed] [Google Scholar]
- MÜNZEL T., HINK U., YIGIT H., MACHARZINA R., HARRISON D.G., MULSCH A. Role of superoxide dismutase in in vivo and in vitro nitrate tolerance. Br. J. Pharmacol. 1999;127:1224–1230. doi: 10.1038/sj.bjp.0702622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MÜNZEL T., LI H., MOLLNAU H., HINK U., MATHEIS E., HARTMANN M., OELZE M., SKATCHKOV M., WARNHOLTZ A., DUNCKER L., MEINERTZ T., FORSTERMANN U. Effects of long-term nitroglycerin treatment on endothelial nitric oxide synthase (NOS III) gene expression, NOS III-mediated superoxide production, and vascular NO bioavailability. Circ. Res. 2000;86:E7–E12. doi: 10.1161/01.res.86.1.e7. [DOI] [PubMed] [Google Scholar]
- MÜNZEL T., SAYEGH H., FREEMAN B.A., TARPEY M.M., HARRISON D.G. Evidence for enhanced vascular superoxide anion production in nitrate tolerance. A novel mechanism underlying tolerance and cross-tolerance. J. Clin. Invest. 1995;95:187–194. doi: 10.1172/JCI117637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURATA T., SATO K., HORI M., OZAKI H., KARAKI H. Decreased endothelial nitric-oxide synthase (eNOS) activity resulting from abnormal interaction between eNOS and its regulatory proteins in hypoxia-induced pulmonary hypertension. J. Biol. Chem. 2002;277:44085–44092. doi: 10.1074/jbc.M205934200. [DOI] [PubMed] [Google Scholar]
- NAGANO T. Practical methods for detection of nitric oxide. Luminescence. 1999;14:283–290. doi: 10.1002/(SICI)1522-7243(199911/12)14:6<283::AID-BIO572>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- NAKANO Y., KUSAMA N., KAJIKURI J., SUZUKI Y., KANMURA Y., ITOH T. Role of PKC in the attenuation of the cGMP-mediated relaxation of skinned resistance artery smooth muscle seen in glyceryl-trinitrate-tolerant rabbit. Br. J. Pharmacol. 2004;141:391–398. doi: 10.1038/sj.bjp.0705625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKATSUBO N., KOJIMA H., KIKUCHI K., NAGOSHI H., HIRATA Y., MAEDA D., IMAI Y., IRIMURA T., NAGANO T. Direct evidence of nitric oxide production from bovine aortic endothelial cells using new fluorescence indicators: diaminofluoresceins. FEBS Lett. 1998;427:263–266. doi: 10.1016/s0014-5793(98)00440-2. [DOI] [PubMed] [Google Scholar]
- PALMER R.M., ASHTON D.S., MONCADA S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- PARKER J.D., FARRELL B., FENTON T., COHANIM M., PARKER J.O. Counter-regulatory responses to continuous and intermittent therapy with nitroglycerin. Circulation. 1991;84:2336–2345. doi: 10.1161/01.cir.84.6.2336. [DOI] [PubMed] [Google Scholar]
- PARKER J.O., PARKER J.D., CALDWELL R.W., FARRELL B., KAESEMEYER W.H. The effect of supplemental L-arginine on tolerance development during continuous transdermal nitroglycerin therapy. J. Am. Coll. Cardiol. 2002;39:1199–1203. doi: 10.1016/s0735-1097(02)01729-1. [DOI] [PubMed] [Google Scholar]
- PITTNER J., LIU R., BROWN R., WOLGAST M., PERSSON A.E. Visualization of nitric oxide production and intracellular calcium in juxtamedullary afferent arteriolar endothelial cells. Acta Physiol. Scand. 2003;179:309–317. doi: 10.1046/j.1365-201X.2003.01137.x. [DOI] [PubMed] [Google Scholar]
- QUIJANO C., HERNANDEZ-SAAVEDRA D., CASTRO L., MCCORD J.M., FREEMAN B.A., RADI R. Reaction of peroxynitrite with Mn-superoxide dismutase. Role of the metal center in decomposition kinetics and nitration. J. Biol. Chem. 2001;276:11631–11638. doi: 10.1074/jbc.M009429200. [DOI] [PubMed] [Google Scholar]
- RAMAN C.S., LI H., MARTASEK P., KRAL V., MASTERS B.S., POULOS T.L. Crystal structure of constitutive endothelial nitric oxide synthase: a paradigm for pterin function involving a novel metal center. Cell. 1998;95:939–950. doi: 10.1016/s0092-8674(00)81718-3. [DOI] [PubMed] [Google Scholar]
- SHIMIZU S., YASUDA M., ISHII M., NAGAI T., KIUCHI Y., YAMAMOTO T. Stimulation of in vitro angiogenesis by tetrahydrobiopterin in bovine aortic endothelial cells. Jpn. J. Pharmacol. 1999;80:177–180. doi: 10.1254/jjp.80.177. [DOI] [PubMed] [Google Scholar]
- SOFF G.A., CORNWELL T.L., CUNDIFF D.L., GATELY S., LINCOLN T.M. Smooth muscle cell expression of type I cyclic GMP-dependent protein kinase is suppressed by continuous exposure to nitrovasodilators, theophylline, cyclic GMP, and cyclic AMP. J. Clin. Invest. 1997;100:2580–2587. doi: 10.1172/JCI119801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWART D.J., ELSNER D., SOMMER O., HOLTZ J., BASSENGE E. Altered spectrum of nitroglycerin action in long-term treatment: nitroglycerin-specific venous tolerance with maintenance of arterial vasodepressor potency. Circulation. 1986;74:573–582. doi: 10.1161/01.cir.74.3.573. [DOI] [PubMed] [Google Scholar]
- STEWART D.J., HOLTZ J., BASSENGE E. Long-term nitroglycerin treatment: effect on direct and endothelium-mediated large coronary artery dilation in conscious dogs. Circulation. 1987;75:847–856. doi: 10.1161/01.cir.75.4.847. [DOI] [PubMed] [Google Scholar]
- STROES E.S.G., VAN FAASSEN E.E., YO M., MARTASEK P., BOER P., GOVERS R., RABELINK T.J. Folic acid reverts dysfunction of endothelial nitric oxide synthase. Circ. Res. 2000;86:1129–1134. doi: 10.1161/01.res.86.11.1129. [DOI] [PubMed] [Google Scholar]
- TOULLEC D., PIANETTI P., COSTE H., BELLEVERGUE P., GRAND-PERRET T., AJAKANE M., BAUDET V., BOISSIN P., BOURSIER E., LORIOLLE F. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J. Biol. Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- TOUYZ R.M., SCHIFFRIN E.L. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol. Rev. 2000;52:639–672. [PubMed] [Google Scholar]
- VÁSQUEZ-VIVAR J., KALYANARAMAN B., MARTASEK P., HOGG N., MASTERS B.S., KAROUI H., TORDO P., PRITCHARD K.A.Jr. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc. Natl. Acad. Sci. U.S.A. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERHAAR M.C., STROES E., RABELINK T.J. Folates and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2002;22:6–13. doi: 10.1161/hq0102.102190. [DOI] [PubMed] [Google Scholar]
- VERHAAR M.C., WEVER R.M., KASTELEIN J.J., VAN DAM T., KOOMANS H.A., RABELINK T.J. 5-Methyltetrahydrofolate, the active form of folic acid, restores endothelial function in familial hypercholesterolemia. Circulation. 1998;97:237–241. doi: 10.1161/01.cir.97.3.237. [DOI] [PubMed] [Google Scholar]
- WEVER R.M., VAN DAM T., VAN RIJN H.J., DE GROOT F., RABELINK T.J. Tetrahydrobiopterin regulates superoxide and nitric oxide generation by recombinant endothelial nitric oxide synthase. Biochem. Biophys. Res. Commun. 1997;237:340–344. doi: 10.1006/bbrc.1997.7069. [DOI] [PubMed] [Google Scholar]
- WHITE C.R., MOELLERING D., PATEL R.P., KIRK M., BARNES S., DARLEY-USMAR V.M. Formation of the NO donors glyceryl mononitrate and glyceryl mononitrite from the reaction of peroxynitrite with glycerol. Biochem. J. 1997;328:517–524. doi: 10.1042/bj3280517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODMAN R.J., CELERMAJER D.E., THOMPSON P.L., HUNG J. Folic acid does not improve endothelial function in healthy hyperhomocysteinaemic subjects. Clin. Sci. (London) 2004;106:353–358. doi: 10.1042/CS20030296. [DOI] [PubMed] [Google Scholar]
- YOSHIMURA T., YOKOYAMA H., FUJII S., TAKAYAMA F., OIKAWA K., KAMADA H. In vivo EPR detection and imaging of endogenous nitric oxide in lipopolysaccharide-treated mice. Nat. Biotechnol. 1996;14:992–994. doi: 10.1038/nbt0896-992. [DOI] [PubMed] [Google Scholar]
- ZELIS R., MASON D.T. Isosorbide dinitrate. Effect on the vasodilator response to nitroglycerin. JAMA. 1975;234:166–170. doi: 10.1001/jama.234.2.166. [DOI] [PubMed] [Google Scholar]