Abstract
Certain fatty acid amides such as anandamide (AEA) and olvanil are agonists for the transient receptor potential, vanilloid-1 (TRPV1) receptor, but have been found to activate TRPV1-containing C-fibers in some tissues but not others. We used extracellular recording and whole-cell patch clamp techniques to investigate the effect of olvanil and AEA on different types of vagal C-fibers innervating the same tissue, namely jugular and nodose vagal C-fibers in guinea pig lungs.
A 30 s exposure to AEA and olvanil caused action potential discharge in all nodose C-fiber innervating lung but failed to activate jugular C-fibers innervating lung and airways. The activation of nodose C-fibers was blocked by the TRPV1 antagonist iodo-resiniferatoxin.
In whole-cell patch clamp recordings of dissociated nodose and jugular capsaicin-sensitive neurons labeled from lungs and airways, olvanil induced large TRPV1-dependent inward currents in cell bodies of both nodose and jugular ganglion neurons.
Prolonged exposure (up to 5 min) to olvanil caused action potential discharge in jugular C-fiber innervating lung but the onset latency was four times longer in jugular than in nodose C-fibers. The onsets of capsaicin response in nodose and jugular C-fibers were not different.
Decreasing the tissue temperature to 25°C increased the onset latency of olvanil-induced activation of nodose C-fibers 2–3-fold, but did not effect the latency of the capsaicin response.
Capsaicin, olvanil, and AEA stimulate jugular C-fibers leading to tachykinergic contractions of isolated bronchi. The time to reach half-maximum is more than four times longer for olvanil and AEA, as compared to capsaicin in evoking contractions.
We conclude that brief exposure to certain fatty acid amides, such as AEA and olvanil activate nodose but not jugular C-fiber terminals in the lungs. We hypothesize that this is because the nodose C-fiber terminals are equipped with a temperature-dependent mechanism for effectively and rapidly transporting the TRPV1 agonists so that they gain access to the intracellular binding sites on TRPV1. This transport mechanism may be differently expressed in two distinct subtypes of pulmonary C-fiber terminals innervating the same tissue.
Keywords: TRPV1, anandamide, olvanil, capsaicin, C-fiber, pulmonary, airways, vagus, sensory, extracellular recordings
Introduction
Various fatty acid amides including the endocannabinoid n-arachidonyl ethanolamide (anandamide, AEA), n-arachidonyldopamine, and the synthetic oleic acid homolog of capsaicin, olvanil have been shown to be effective agonists of transient receptor potential, vanilloid-1 (TRPV1) (Szallasi & Di marzo, 2000; Van Der Stelt & Di marzo, 2004). Some fatty acid derivatives appear to act as intracellular messengers between G-protein-coupled receptor stimulation and TRPV1 activation (Hwang et al., 2000). It has also been hypothesized that some of these substances may serve as autacoid mediators of C-fiber activation (Van Der Stelt & Di marzo, 2004).
For these substances to act as C-fiber-activating autacoids, they must be released in the vicinity of sensory C-fiber terminals at concentrations and time periods sufficient to activate the receptor. The concentrations of these lipid mediators required to activate C-fibers are typically large, and seemingly dependent on the tissue in which the C-fiber resides. Even within the same species, the response to these agonists reveals striking differences among the C-fibers located within different tissues (Andersson et al., 2002; Stebbins et al., 2003). In guinea pigs, capsaicin, AEA, and olvanil are all potent and effective in evoking neuropeptide release from mesenteric arterial C-fibers resulting in relaxation of the smooth muscle therein (Zygmunt et al., 1999; Andersson et al., 2002). Capsaicin also potently activates C-fibers within the guinea pig isolated bronchus causing near maximal, tachykinin-dependent tissue contractions. AEA and olvanil can cause tachykinergic contractions of guinea pig airway tissue, but they are orders of magnitude less potent than capsaicin in this regard (Zygmunt et al., 1999; Tucker et al., 2001). Consistent with these findings, Stebbins et al. (2003) found that capsaicin causes action potential discharge in guinea pig tracheal C-fibers, whereas olvanil, even at relatively large concentrations, was ineffective. These observations suggest that the ability of these fatty acid amides to act in a paracrine fashion to activate sensory C-fibers via TRPV1 may be dependent on the C-fiber subtype or the tissue in which the C-fiber is situated.
There are several potential explanations for the differential responses of fatty acid amide TRPV1 agonists among different tissues. Although there is no data to support the existence of more than one TRPV1 gene, it is conceivable that differences in the subunit assembly of the receptor may be different among differing C-fiber populations (Szallasi & Blumberg, 1999). Another consideration is that the vanilloid binding site is located on the intracellular domain of TRPV1 (Jung et al., 1999; Jordt & Julius, 2002). This means that an exogenously applied agonist must first cross the terminal membrane before it can activate the receptor. There is compelling evidence that the transport of fatty acid amide TRPV1 agonists across the nerve membrane may be facilitated by specific proteins (Szallasi & Di marzo, 2000; De Petrocellis et al., 2001). It has been hypothesized, based on studies using a putative AEA uptake antagonist, that differential expression of these putative transporters among C-fibers explains the potency disparities of AEA and olvanil among C-fibers in different tissues (Andersson et al., 2002). Finally, differences in experimental design, methods of agonist delivery, metabolic breakdown of the agonist, as well as the read-out of C-fiber activation (bronchial contractions, arterial relaxation, action potential discharge, etc.) could also substantively influence the observed potency of fatty acid amides in activating C-fibers in different tissues.
We have recently described two distinct vagal C-fiber phenotypes innervating the guinea pig respiratory system (Undem et al., 2004). The C-fibers innervating the guinea pig trachea and bronchus, tissues that have been shown to be very sensitive to capsaicin and resiniferatoxin but poorly responsive to AEA and olvanil, are derived nearly exclusively from cell bodies situated in the jugular ganglia. By contrast, the C-fibers within the lungs are derived from both jugular ganglion neurons, and also from neurons with cell bodies situated in the nodose ganglia. The jugular and nodose C-fiber populations can be differentiated based on embryological background, neuropeptides content, and pharmacological responsiveness (Undem et al., 2004). By using an isolated lung preparation, we were able to investigate the relative responsiveness of two C-fiber phenotypes to TRPV1 agonists using the identical experimental design. The intrapulmonary jugular and intrapulmonary nodose C-fibers were equally responsive to capsaicin as quantified by extracellular recordings of action potential discharge. We report differences between these two types of C-fibers, however, with respect to their responsiveness to fatty acid amide agonists of TRPV1.
Methods
All experiments were approved by the Johns Hopkins Animal Care and Use Committee. Male Hartley guinea pigs (100–300 g, Hilltop Laboratory Animals, Inc., Scottsdale, PA, U.S.A.) were used.
Extracellular recordings
The methods for extracellular recording from the lung preparation have been described in detail previously (Undem et al., 2004). Briefly, the animals were killed by 100% CO2 asphyxiation and followed by exsanguinations. The airways and lungs with their intact right-side extrinsic innervation (including nodose and jugular ganglia) were dissected and placed in a dissecting dish containing Krebs' bicarbonate buffer solution composed of (mM) 118 NaCl, 5.4 KCl, 1.0 NaH2PO4, 1.2 MgSO4, 1.9 CaCl2, 25 NaHCO3, and 11.1 dextrose, and equilibrated with 95% O2 and 5% CO2 (pH 7.2–7.4). Excess connective tissues were trimmed away leaving the larynx, trachea, and right lung with their intact nerves. The larynx, trachea, and lung were pinned to a silicone elastomer (Sylgard) lined Perspex chamber. PE60 tubing was inserted into the trachea and the pulmonary artery for superfusion (2–3 ml min−1) of the buffer and drugs during an experiment. The portion of vagus nerve containing the right nodose and jugular ganglia was gently manipulated into an adjacent compartment of the same chamber through a small hole. The ganglia were pinned to the floor of the chamber. Both compartments were superfused with the buffer solution (37°C or, for some experiments, 25°C) at a flow rate of 6 ml min−1. The buffer solution superfusing the ganglia was kept at 37°C for all experiments.
Sharp glass micropipettes were fabricated using a Flaming Brown micropipette puller (P-87, Sutter Instrument, Novato, CA, U.S.A.) and filled with 3 M NaCl solution. The micropipette electrode (tip resistance ∼1 MΩ) was gently inserted into either nodose or jugular ganglion. The recorded signals were amplified (Microelectrode AC amplifier 1800, A-M system, Everett, WA, U.S.A.), filtered (0.3 kHz of low cutoff and 1 kHz of high cutoff), and monitored on an oscilloscope (TDS340, Tektronix, Beaverton, OR, U.S.A.) and a chart record (TA240, Gould, Valley View, OH, U.S.A.). The scaled output from the amplifier was captured and analyzed by a Macintosh computer using NerveOfIt software (Phocis, Baltimore, MD, U.S.A.). The scaled output was also stored on a digital tape for off-line analysis.
A single mechanically sensitive nerve was identified by probing the lung surface with receptive a von Frey filament (1500–3000 mN). For the majority of experiments, only a single nerve was recorded. Occasionally two fibers were simultaneously recorded, and the individual units were discriminated off-line using the wave-analysis software. For measuring conduction velocity, an electrical stimulation (S44, Grass instrument, Quincy, MA, U.S.A.) was applied on the core of the receptive field. The conduction velocity was calculated by dividing the distance along the nerve pathway by the time delay between the shock artefact and the action potential evoked by electrical stimulation. Only C-fibers (conduction velocity <1 m s−1) were studied.
Olvanil (10−8–10−5 M), capsaicin (3 × 10−7 or 10−6 M), and iodo-resiniferatoxin (I-RTX, 10−7 M) were diluted from a stock solution (10−2 M, dissolved in ethanol) with the buffer solution. The drugs (1 ml) were injected through the pulmonary artery for 30 s. When the olvanil was repeatedly applied, the intervals between them were at least 15 min. Capsaicin was always the last substance to be tested in a given experiment. If the nerve did not respond to capsaicin, it was excluded from the data. Only one nerve fiber per lung preparation was studied.
Patch clamp recordings
All nodose and jugular ganglionic neurons studied with patch clamp recordings were previously retrogradely labeled with DiI (DiC18(3); Molecular Probes, Eugene, OR, U.S.A.). The animals were anesthetized with 50 mg kg−1 of ketamine and 2.5 mg kg−1 of xylazine, i.p. The DiI solution (0.2%, 0.3 ml; dissolved in 10% of DMSO and 90% of normal saline) was then injected into the tracheal lumen using a 27G needle. Post-mortem analysis confirmed that the dye was found exclusively in the pulmonary tissues. After 7–10 days, the nodose and jugular ganglia were rapidly dissected from the animal, cleared of adhering connective tissue, and incubated in an enzyme solution (10 mg of collagenase type 1A and 10 mg of dispase II in 10 ml of Ca2+-, Mg2+-free Hanks' balanced salt solution) for 2 h at 37°C. Neurons were dissociated by trituration with glass pasteur pipettes of three gradually decreasing tip pores. Then, they were washed by centrifugation (three times at 700 × g for 45 s) and suspended in L-15 medium containing 10% fetal bovine serum (bovine serum albumin, BSA). Cell suspensions were transferred onto circular 15 mm glass coverslips (Bellco Glass Inc., Vineland, NJ, U.S.A.) coated with poly-D-lysine (0.1 mg ml−1). After the suspended neurons adhered to coverslips in 2 h, the neuron-attached coverslips were flooded with the L-15 medium (10% of BSA) and stored at 37°C and used within 24 h.
The labeled cells were identified with fluorescent microscopy equipped with 560 nm of excitation filter and 480 nm of emission filter. A conventional technique for a whole-cell patch clamp recording was employed using an Axoclamp 200A amplifier and pCLAMP7 software (Axon Instruments, Union City, CA, U.S.A.). Pipettes (1.5–3 MΩ) were filled with a solution composed of (in mM): 140 KCl, 1 CaCl2, 2 MgCl2, 10 HEPES, 11 EGTA, and 10 dextrose; titrated to pH 7.3 with KOH; 304 mOsm. The membrane potentials of the cells were held at −60 mV. After the electrical characteristics of the cell membrane were determined by a depolarizing test pulse of 5 mV, the membrane capacitance (Cm) and 60–80% of series resistance (Rs) were compensated. Criteria for cell inclusion in the study were Rs <10 MΩ and input resistance of >100 MΩ. During the experiments, the cells were continuously superfused (3 ml min−1) by gravity with Locke solution (35°C); composition (mM): 136 NaCl, 5.6 KCl, 1.2 MgCl2, 2.2 CaCl2, 1.2 NaH2PO4, 14.3 NaHCO3, and 10 dextrose (pH 7.3–7.4).
Olvanil (10−6 M), capsaicin (10−6 M), I-RTX (10−7 and 10−6 M) were diluted in the Locke solution from the stock solution and applied into a recording bath by switching the perfusion flow to drug-containing Locke solution. A neuron was considered unresponsive to a drug if the inward current failed to exceed 100 pA. The intervals between drug applications were at least 5 min. The peak inward and the total inward current per unit membrane capacitance were measured.
Efferent function of jugular C-fibers
Guinea pigs were killed by asphyxiation with CO2 and exsanguinated. The main stem bronchi were removed, trimmed of excess tissue, placed in tissue baths and tied with silk surgical suture to force–displacement transducers (FT03C, Grass Instrument Co., Quincy, MA, U.S.A.) for recording of isometric tension on a Grass polygraph, as described elsewhere (Undem & Kollarik, 2002). Resting tension was set at 1 g. Tissue baths contained 10 ml of the Kreb's bicarbonate solution maintained at 37°C and gassed with 95% O2–5% CO2 and replaced every 15 min during a 60-min equilibration period.
After the equilibration period, cumulative concentration–response curves were obtained with capsaicin, olvanil, or anandamide. The contractions were analyzed as a percentage of the maximum contraction obtained with methacholine (1 mM) added at the end of the experiment. In some studies, a single, maximally or submaximally effective concentration of the agonist was added and the time for the contraction to reach 50% of its maximum was noted. In four studies, we found that the contractions to capsaicin, olvanil, and AEA were abolished by the combined antagonism of neurokinin (NK)1 and NK2 receptors with SR 140333 and SR 48968, respectively (1 μM each).
Data analysis
In the extracellular recording studies, the action potential discharge was quantified off-line and recorded in 1 s bins. A response was considered positive if the number of action potentials in any 1 s bin was >2 × the average background response. The background activity was usually either absent or less than 2 Hz. The total number of action potentials was that number of action potentials that occurred between the onset of action and the point at which the response waned to <2 × background. The peak frequency evoked by a stimulus was quantified as the maximum number of action potentials that occurred within any 1 s bin. The data were expressed as a mean±s.e.m. Student's t-test, paired t-test, and χ2-test were used when appropriated. The P-value under 0.05 was considered statistically significant.
Results
Electrophysiology
Extrapulmonary C-fibers
The vagal C-fibers in the extrapulmonary airways are derived from neurons situated in the jugular ganglia. Applying 1 ml of either AEA (30 μM × 30 s, n=5) or olvanil (1 μM × 30 s, n=6) directly to the receptive field in the tracheal/bronchial wall did not evoked action potential discharge from any jugular C-fiber studied. By contrast, capsaicin vigorously activated all C-fibers with action potential discharge of >5 Hz. The lack of effect of olvanil on tracheal C-fibers is consistent with previous reports (Stebbins et al., 2003).
Intrapulmonary C-fibers
The vagal C-fibers in guinea pig intrapulmonary tissue are derived from cell bodies situated in the nodose and jugular ganglia. A total of 39 intrapulmonary vagal C-fibers were studied (n=22 nodose C-fibers, n=17 jugular C-fibers). One fiber was studied per animal. All receptive fields were mechanically characterized using von Frey filaments applied to the lung surface; the surface distributions of these receptive fields were similar between nodose and jugular type C-fibers as we previously reported (Undem et al., 2004). All jugular and nodose C-fibers responded to capsaicin with action potential discharge (capsaicin was added at the end of the experiment to avoid issues of desensitization).
Olvanil (1 μM) application (1 ml, 30 s via the pulmonary artery) activated all (17/17) intrapulmonary nodose C-fibers causing the discharge of 100±28 action potentials. Likewise, AEA (10 μM) activated all (9/9) intrapulmonary nodose C-fibers causing an average of 100±30 action potentials (Figure 1a). These responses were similar in magnitude with that observed with capsaicin (0.3 μM, 137±34 action potentials).
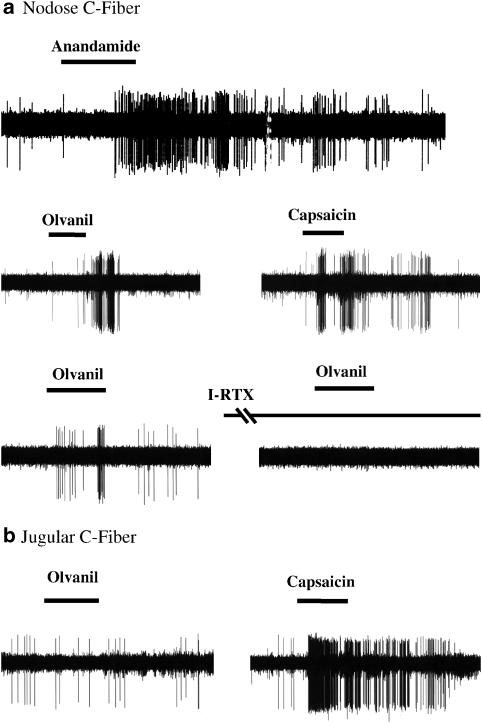
Figure 1.
Extracellular recordings of intrapulmonary nodose and jugular C fibers in isolated lung preparation. (a) A 30 s infusion with either anandamide (10 μM) or olvanil (Olv, 1 μM) evoked action potential discharge in a nodose capsaicin-sensitive C-fiber. Iodo-resiniferatoxin (I-RTX, 1 μM for 20 min), a selective TRPV1 antagonist, completely blocked the response evoked by 30 s infusion with olvanil in an intrapulmonary nodose C fiber. (b) By contrast, a 30 s infusion with Olvanil typically did not evoke action potential discharge in jugular C-fibers. All 17 tested nodose C fibers but only two of 12 jugular C fibers responded to olvanil. Two different subtypes of vagal C-fibers showed difference in olvanil sensitivity (P<0.001, χ2-test).
We next addressed possible mechanisms for this difference. We focused the remainder of the studies on olvanil, because this agonist is less susceptible to differences caused by differential expression of fatty acid hydrolases, or cannabinoid receptors (Di Marzo et al., 1998).
We first addressed whether the difference in olvanil responsiveness was due to C-fiber type (nodose vs jugular C-fibers) or because of tissue differences (lung vs trachea). We evaluated 12 jugular C-fibers with receptive fields within the lungs. Using the identical experimental design as that used to study intrapulmonary nodose C-fibers described above, we found that 10 of 12 jugular C-fibers failed to respond to 30 s application of olvanil. All fibers responded to capsaicin added at the end of the experiment. There was a significant difference in the responsiveness of intrapulmonary jugular vs intrapulmonary nodose C-fiber to olvanil (Figure 1b, χ2-test, P<0.001).
We evaluated whether olvanil was activating intrapulmonary nodose C-fibers by some indirect mechanism that was independent of TRPV1. We obtained two consecutive olvanil responses separated by 20 min exposure to either vehicle or I-RTX (0.1 μM). Following 20 min of vehicle treatment, the second olvanil response was 78±19% of the first one in the number of action potentials (n=7). Following 20 min treatment with I-RTX (0.1 μM, Figure 1a), the olvanil response was abolished.
Patch clamp recordings
The above data suggest that the TRPV1 in jugular C-fibers is relatively insensitive to olvanil, whereas the TRPV1 in nodose C-fibers is stimulated by olvanil. We next used whole-cell patch clamp recording to study the membrane properties of isolated nodose and jugular neurons retrogradely labeled from the lungs. A total 33 respiratory labeled neurons were studied, 22 isolated from nodose ganglia and 11 isolated from jugular ganglia. Only capsaicin-sensitive cell bodies were studied. The capsaicin sensitive neurons were 10 of 11 and 13 of 22 in the jugular and the nodose ganglia, respectively. The diameters of the capsaicin-sensitive neurons were 30.5±1.8 and 31.5±1.1 μm, and the cell capacitances were 35.4±5.8 and 48.4±6.6 pF in the jugular and the nodose neurons, respectively (P>0.1).
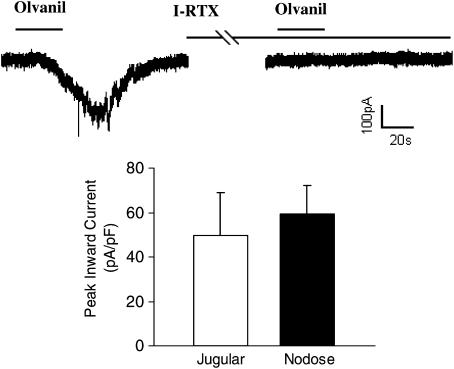
By contrast to the effect of olvanil on nerve terminals, nodose and jugular capsaicin-sensitive respiratory-specific cell bodies were equally responsive to olvanil. We found that eight of 10 (80%) jugular capsaicin (1 μM)-sensitive neurons and 12 of 13 (92%) nodose neurons responded to olvanil with a rapid inward current (Figure 2). The peak inward currents induced by olvanil (1 μM, 30 s application) were similar between jugular and nodose neurons, averaging 49.6±19.3 and 59.3±12.8 pA/pF, respectively. The total inward currents were 1.52±0.63 and 1.40±0.43 nA s pF−1 (P>0.1) in the jugular and the nodose ganglia, respectively. The inward currents induced by olvanil were completely blocked by a selective TRPV1 antagonist, I-RTX (1 μM, 5 min) in both jugular and nodose ganglia (each n=3, Figure 2).
Figure 2.
Whole-cell patch clamp recording of an olvanil-induced inward current in an airway-labeled jugular neuron. Olvanil (1 μM, 30 s) evoked inward currents in 12 of 13 nodose and eight of 10 jugular airway-labeled capsaicin-sensitive neurons. The histogram shows that the peak inward current induced by olvanil were similar between nodose and jugular capsaicin-sensitive neurons. The recording also shows that the olvanil-induced current in the jugular neuron was blocked by I-RTX (1 μM, 5 min). The same result was obtained in nodose neurons (n=3).
Effect of exposure duration and temperature
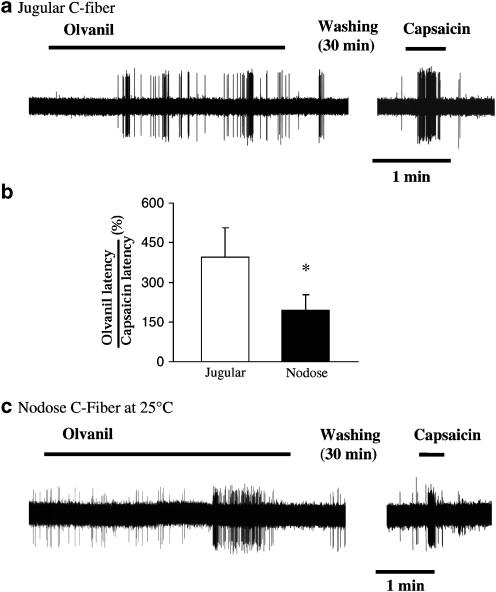
The patch clamp data suggest that both jugular and nodose C-fiber neurons express functional olvanil-sensitive TRPV1 receptors. We therefore reasoned that notable differences in the response of nodose vs jugular C-fiber terminals in the tissue are most likely due to differences in accessibility of the agonist. We carried out a series of studies in which we increased the exposure duration of olvanil from 30 s to >5 min. The more prolonged exposure led to the activation of seven of eight jugular C-fibers. Each of these seven fibers had a long onset latency averaging 45±7 s (Figure 3a and b). The onset latency for olvanil responses in nodose C-fibers was only 22±2 s (P<0.05, compared to jugular C-fiber responses). Prolonged treatment with olvanil failed to evoke action potential discharge in jugular C-fibers when the lungs were pretreated with I-RTX (0.1 μM, n=2).
Figure 3.
The latency of response to olvanil. (a) Prolonged exposure of olvanil (1 μM, >1 min) evoked action potential discharge with long latency of response in jugular C fibers (n=5). (b) The histogram shows that onset latency of the olvanil response in intrapulmonary jugular C-fibers, relative to the latency of the capsicin response, was two-fold longer than that observed for intrapulmonary C-fibers (*P<0.05; n= 5 and 8 for jugular and nodose responses, respectively). (c) Representative olvanil response showing the delayed onset in a nodose C fiber in tissue studied at 25°C. Decreasing temperature prolonged the onset latencies of olvanil response in nodose C fibers (see text for details).
The latency of response is the combination of the time it takes for the infused agonist to reach the receptive field within the lungs, and the time it takes to activate TRPV1 once it reaches the receptive field. We have previously found that capsaicin, when studied in extrapulmonary airways (trachea and main bronchus) where the drug can be applied directly to the receptive field, evokes action potential discharge with an onset time of <2 s. We therefore used the onset time of capsaicin in each experiment as an attempt to normalize for time of diffusion to the receptive field. The onset time of response for infused capsaicin was not different between jugular and nodose C-fibers and averaged 13.0±3.6 and 19.6±4.5 s, respectively (P>0.1). The percentage of olvanil onset to capsaicin onset averaged 195±59% in nodose C-fibers. By contrast, it took olvanil about four times longer than capsaicin to activate jugular C-fibers with the percentage averaging 395±112% (Figure 3b, P<0.05 compared to the ratio obtained in the nodose C-fiber). The long onset time for olvanil clearly resulted in ‘false negative' responses when, as described above, olvanil was studied as a 1 ml (30 s) exposure.
We quantified the onset times of olvanil relative to capsaicin in the jugular and nodose C-fiber cell bodies using whole-cell patch clamp techniques. Unlike the studies at the terminals, the percentage of olvanil (1 μM) onset time to capsaicin (1 μM) onset time in nodose and jugular ganglionic neurons were 96.0±10.0% (n=5) and 92.8±10.8% (n=6), respectively.
The effect of decreasing the temperature of the buffer solution perfusing the tissue to 25°C (for 20 min) was tested in six nodose C-fibers. As expected, decreasing the tissue temperature decreased the effectiveness of the fiber to TRPV1 stimulation. At 25°C, capsaicin (1 μM) evoked 28±5 action potentials. The key variable in these studies, however, was not efficacy, but onset latency. The onset time of the capsaicin response was 10±2 s, which was not different than that observed at 37°C (P>0.1). Five of six nodose C-fibers studied at 25°C responded to olvanil (Figure 3c). The latency of response to olvanil at 25°C averaged 62±28 s (P<0.05 compared to 37°C). The olvanil response latency in nodose fibers studied at 25°C was similar to that seen with jugular C-fibers studied at 37°C (41±11 s).
A facilitated transport mechanism using a so-called AEA transporter has been proposed as a mechanism by which AEA and olvanil (but not capsaicin) gains entry into cells (Szallasi & Di marzo, 2000; De Petrocellis et al., 2001). We evaluated the effect of the AEA transport inhibitor VDM11 on the olvanil response in nodose C-fibers. At effective concentrations, 10 and 30 μM, this compound had nonselective effects in that it evoked a modest number of action potentials, and blocked (30 μM) or inhibited (10 μM) the response of the fiber to capsaicin. Owing to these effects, we were not able to effectively use VDM11 as a selective transport inhibitor in our experimental design.
AEA is a CB1/CB2 cannabinoid receptor agonist. Olvanil has inconsistently been found to be a CB1 receptor agonist (Di Marzo et al., 1998; Appendino et al., 2005). It is possible that the lack of effect of olvanil and AEA on jugular C-fibers is due an inhibitory effect of simultaneous cannabinoid receptor activation. To address this hypothesis, we evaluated the effect of olvanil (1 μM × 30 s) on jugular C-fibers in the trachea in the presence of the CB1 receptor antagonist, AM-281 (1 μM × 30 min). In five of six tissues treated with AM-281, olvanil failed to evoke action potential discharge. In a separate series of studies, we examined the effect of the CB1/CB2 cannabinoid receptor agonist CP 55940 on citric acid-induced action potential discharge in jugular C-fibers. Citric acid (1 mM, 30 s) was chosen as this can evoke responses that are partially TRPV1 dependent, but unlike capsaicin, citric acid acts in a nondesensitizing manner. The intrapulmonary jugular C-fiber response to citric acid was 117±23 action potentials at a peak frequency of 32±4 Hz (n=3). After 10 min of treatment with a large concentration of CP 55940 (30 μM), the response in the same fibers did not change (116±16 action potentials, peak frequency 32±5 Hz).
Efferent function (tachykinergic contractions of isolated bronchi)
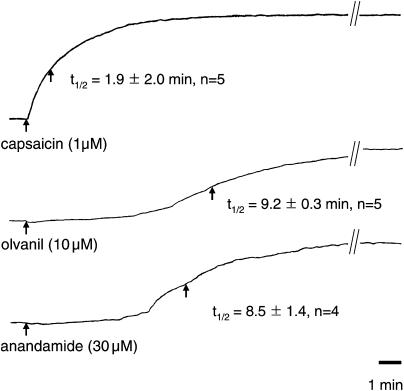
It is well known that TRPV1 agonists can evoke contractions of guinea pig isolated airways. We have previously found that the tachykinergic fibers in the trachea and bronchi responsible for these responses are jugular C-fibers (Riccio et al., 1996). We, and others, have reported that AEA causes tachykinergic contractions of the guinea pig isolated bronchus (Craib et al., 2001; Tucker et al., 2001; Undem & Kollarik, 2002). In our studies, the maximum response was found to be similar to that obtained with capsaicin, but with an EC50 (∼ 30 μM) about 1000-fold larger than the EC50 for capsaicin (∼30 nM). In the present study, as expected, we found that olvanil contracted the guinea pig bronchus by a mechanism that was abolished by combined treatment with NK-1 and NK-2 receptor antagonists (SR 140333 and SR 48968, 1 μM each, n=4). The maximum response averaged 62±2% and the –log (M) EC50 averaged 5.5±3 (i.e., about 100-fold less potent than capsaicin). We evaluated the kinetics of the contractile response to a single large concentration of capsaicin, olvanil, and AEA (Figure 4). Capsaicin (0.1 μM) caused a contraction that averaged 64±5% of the tissue maximum. The time to reach 50% of the peak response (t1/2) averaged 1.9±0.2 min. A large and significant difference between capsaicin and olvanil was, again, seen in the kinetics of the response. Olvanil (30 μM) caused contractions that averaged 62±2% of the tissue maximum, and AEA (30 μM) caused 40±4% contraction. The t1/2 of the fatty acid amide TRPV1 agonists were 4–5 times longer than for capsaicin (P<0.01 compared to capsaicin, Figure 4). Treating the tissues with the CB1 receptor antagonist (AM281, 1 μM) had no significant effect on the magnitude or kinetics of the olvanil- or anandamide-induced contractions (n=4, P>0.1, data not shown).
Figure 4.
Representative traces of guinea pig isolated bronchial contractions to capsaicin, olvanil, and anandamide. These contractions are caused by tachykinin release (see text for details). The numbers in parentheses represent the time required to reach 50% of the maximum response (t1/2). The t1/2 was significantly different between capsaicin and either olvanil or anandamide (P<0.01). The values represent the mean±s.e.m. of n experiments with each experiment carried out on a tissue from different animals.
Discussion
There are two different types of vagal C-fibers innervating the pulmonary system. One type of C-fiber is derived from the jugular ganglia, the other from the nodose ganglia. We have previously demonstrated that these two subtypes of C-fibers in guinea pig lungs can be distinguished based on neurochemistry and in their response to various chemical activators (Undem et al., 2004, Chuaychoo et al., 2005). Here, we show that these two C-fiber phenotypes can also be differentiated based on their relative responsiveness to certain fatty acid-amide TRPV1 agonists. We conclude that transient exposure to AEA and olvanil are more effective in stimulating TRPV1 to cause action potential discharge in the terminals of nodose (placode-derived) C-fibers as compared to jugular (neural crest-derived) C-fibers. The observations by other laboratories that C-fibers in the guinea pig bronchus and trachea are relatively unresponsive to olvanil and/or AEA (Tucker et al., 2001; Andersson et al., 2002; Stebbins et al., 2003) are consistent with the fact that these C-fibers are jugular C-fibers (Riccio et al., 1996).
Nodose C-fibers within the isolated lung were found to be more responsive to 30 s exposure to olvanil than jugular C-fibers found in the trachea or bronchus. This is unlikely explained simply by differences in tissue type (cartilaginous airway vs lung) or experimental design differences, because we also noted that the jugular C-fibers within the lung are much less responsive to olvanil than the nodose C-fibers within the lungs, studied under identical experimental conditions. Rather, our data support the hypothesis that there may be substantive differences in agonist access to the TRPV1 receptor within the terminals of the two C-fiber subtypes. In this regard, we are in agreement with the conclusion of Andersson et al. (2002), who concluded that the differences in the degree of transport of the agonist across the membrane explain why olvanil and AEA are such more potent in activating C-fibers in mesenteric arteries as compared to bronchial tissue.
Whole-cell patch clamp recording of capsaicin-sensitive neurons, retrogradely labeled with dye placed in the lungs, was used to directly address the issue of TRPV1 subtypes in the two C-fiber phenotypes. Unlike the studies on nerve terminals within the tissue, olvanil was equally effective in inducing inward currents in both jugular and nodose cell bodies. Although extensive concentration–response and kinetic analysis was not carried out, we obtained no evidence of qualitative differences in the TRPV1 response in jugular vs nodose neurons. In both types of neurons, the response to olvanil was similar to that observed with capsaicin. These data argue that if the olvanil molecules reach the active site of TRPV1, the patch clamp data predict that similar activation will occur in both nodose and jugular C-fibers.
It seems unlikely that a failure to reach the jugular C-fiber terminals explains the lack of response to a 30 s olvanil exposure. It is not yet known precisely where within the lung the two types of C-fiber are situated, but it is noteworthy that the sensitivity to capsaicin, and the onset time for capsaicin-induced responses, was not different between the two C-fiber subtypes. Moreover, transient exposure to olvanil and AEA failed to activate jugular C-fiber terminals in the isolated trachea, where the drug can be applied directly to the region of the receptive field.
Olvanil has been reported to activate cannabinoid CB1 receptors and AEA is a CB1/CB2 receptor agonist (Di Marzo et al., 1998; Szallasi & Di marzo, 2000). It is unlikely, however, that the relative lack of response of jugular C-fibers to AEA and olvanil was due to countervailing inhibitory effects of cannabinoid CB1 receptor activation. Andersson et al. (2002) and Tucker et al. (2001) showed that pharmacologically blocking CB1 receptors does not increase the AEA-induced contraction of the isolated bronchus, a jugular C-fiber-mediated response. Consistent with this, we also noted that blocking CB1 receptors had no effect on the kinetics of the tachykinergic contraction of the isolated airway, or on action potential discharge in tracheal jugular C-fibers. Moreover, we found that a large concentration of CP 55940, a CB1/CB2 receptor agonist, did not inhibit citric acid-induced action potential discharge in jugular C-fibers. Citric acid was chosen in these studies because we have previously noted that citric acid activates guinea pig airway jugular C-fibers in part via TRPV1 stimulation, but unlike capsaicin shows little desensitization upon repeated treatments (Undem & Kollarik, 2002).
It may be argued that the reason nodose but not jugular C-fibers rapidly responded to AEA and olvanil is because they express a higher density of TRPV1 receptors than jugular C-fibers. If this were the case, the intrinsic efficacy of an agonist required to cause action potential discharge could theoretically be lower in nodose compared to jugular C-fibers. Arguing against this scenario is the finding that olvanil appears to be a full agonist at guinea pig cloned TRVP1 receptors (Savidge et al., 2002). Moreover, capsaicin evoked quantitatively similar responses in both types of C-fibers (here and Undem et al., 2004), and the peak inward current evoked by olvanil was not quantitatively different, at the level of the cell body, between jugular and nodose C-fibers. Also arguing against this hypothesis is the observation that the major difference in the response of the two C-fiber types was found in the time course (onset of action) and not in the efficacy of the response.
The vanilloid-binding site on TRPV1 is intracellular (Jung et al., 1999; Jordt & Julius, 2002). This means that the vanilloid must first cross the nerve terminal plasma membrane before it can activate the receptor. Several studies have demonstrated that diffusion of fatty-acid amide agonists such as AEA and olvanil can be substantially facilitated by an as of yet unidentified molecular mechanism (Di Marzo et al., 1998; Beltramo & Piomelli, 1999; De Petrocellis et al., 2000, 2001; Hillard & Jarrahian, 2003; Fegley et al., 2004). It is therefore possible that nodose C-fiber neurons express this transport mechanism to a greater extent than jugular C-fibers. To address the issue of accessibility, we carried out experiments in which we continually perfused the receptive fields of jugular C-fibers with olvanil. Under these conditions, jugular C-fibers responded, but with a very long onset time of action relative to nodose C-fibers. This was the case even when the data were normalized to the onset time of the capsaicin response. We reasoned if some transport mechanism accounted for the more rapid response in the nodose terminals, decreasing the temperature would affect the onset of olvanil more than capsaicin (Maccarrone et al., 2000; De Petrocellis et al., 2001; Fegley et al., 2004). Consistent with this argument, we found that decreasing the temperature of the tissue by 10°C had little effect on the onset time for capsaicin, but significantly increased the onset time of olvanil by about 2.5-fold. Indeed, at 25°C, the nodose C-fibers responded to olvanil-like jugular C-fibers studied at 37°C. These observations provide circumstantial support for the hypothesis that in nodose C-fibers olvanil gained rapid access to the TRPV1 receptor by a transport mechanism, and that this putative transporter (mechanism) is less expressed in jugular C-fibers.
The difference in agonist accessibility to TRPV1 noted at the terminals was not observed at the cell body where the onset of the olvanil-induced inward currents was rapid in both jugular and nodose neurons. One possibility is that both types of C-fiber neurons express the transporter in the cell body, but the nerve terminal density of the transport mechanism is much greater in nodose vs jugular fibers. Molecular identification of the putative transporter is needed before this hypothesis can be rigorously addressed. Our attempt to use VDM11 as a tool to address this issue was stymied by the nonselective effects of the compound, a problem that has been noted by others (De Petrocellis et al., 2001).
Savidge et al. (2002) have studied the ability of various TRPV1 agonists to evoke increases in intracellular calcium in CHO cells expressing guinea pig cloned TRPV1. Olvanil was a full agonist in this assay and was found to be about 20 times more potent than capsaicin. In the bronchial contraction assay, we also noted that olvanil acted as a full agonist, but was about 100-fold less potent than capsaicin. The reason for this large difference in relative potencies is not known. The activation of TRPV1 by vanilloid agonists is a balance of activating and inactivating mechanisms (Liu et al., 1997). It is possible that the slow accumulation of olvanil may favor inactivating mechanisms, such that at the lower concentration no action potential discharge or tachykinin secretion is observed.
The results presented here support the hypothesis that as intracellular messengers fatty acid amides will be equally effective in activating both nodose and jugular C-fibers via TRPV1 stimulation. However, when these types of TRPV1 agonists act as extracellular autacoids, where the exposure time to the terminal membranes may be limited, nodose C-fibers will be more likely to be activated than jugular C-fibers.
Acknowledgments
This work was supported by NIH (Bethesda, MD) and also by a Korean Science and Engineering Foundation (KOSEF) grant (M.-G.L.).
Abbreviations
- AEA
arachidonoyl-ethanolamide (anandamide)
- BSA
bovine serum albumin
- EGTA
ethyleneglycol-bis(β-aminoethyl)-N,N,N′,N′-tetraacetic acid
- I-RTX
iodo-resiniferatoxin
- TRPV
transient receptor potential, vanilloid
References
- ANDERSSON D.A., ADNER M., HOGESTATT E.D., ZYGMUNT P.M. Mechanisms underlying tissue selectivity of anandamide and other vanilloid receptor agonists. Mol. Pharmacol. 2002;62:705–713. doi: 10.1124/mol.62.3.705. [DOI] [PubMed] [Google Scholar]
- APPENDINO G., DE PETROCELLIS L., TREVISANI M., MINASSI A., DADDARIO N., MORIELLO A.S., GAZZIERI D., LIGRESTI A., CAMPI B., FONTANA G., PINNA C., GEPPETTI P., DI MARZO V. Development of the first ultra-potent ‘capsaicinoid' agonist at transient receptor potential vanilloid type 1 (TRPV1) channels and its therapeutic potential. J. Pharmacol. Exp. Ther. 2005;312:561–570. doi: 10.1124/jpet.104.074864. [DOI] [PubMed] [Google Scholar]
- BELTRAMO M., PIOMELLI D. Anandamide transport inhibition by the vanilloid agonist olvanil. Eur. J. Pharmacol. 1999;364:75–78. doi: 10.1016/s0014-2999(98)00821-8. [DOI] [PubMed] [Google Scholar]
- CHUAYCHOO B., LEE M.-G., KOLLARIK M., UNDEM B.J. Effect of 5-hydroxytryptamine on vagal C-fiber subtypes in guinea pig lungs. Pulm. Pharmacol. Ther. 2005;18:269–276. doi: 10.1016/j.pupt.2004.12.010. [DOI] [PubMed] [Google Scholar]
- CRAIB S.J., ELLINGTON H.C., PERTWEE R.G., ROSS R.A. A possible role of lipoxygenase in the activation of vanilloid receptors by anandamide in the guinea-pig bronchus. Br. J. Pharmacol. 2001;134:30–37. doi: 10.1038/sj.bjp.0704223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE PETROCELLIS L., BISOGNO T., DAVIS J.B., PERTWEE R.G., DI MARZO V. Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Lett. 2000;483:52–56. doi: 10.1016/s0014-5793(00)02082-2. [DOI] [PubMed] [Google Scholar]
- DE PETROCELLIS L., BISOGNO T., MACCARRONE M., DAVIS J.B., FINAZZI-AGRO A., DI MARZO V. The activity of anandamide at vanilloid VR1 receptors requires facilitated transport across the cell membrane and is limited by intracellular metabolism. J. Biol. Chem. 2001;276:12856–12863. doi: 10.1074/jbc.M008555200. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., BISOGNO T., MELCK D., ROSS R., BROCKIE H., STEVENSON L., PERTWEE R., DE PETROCELLIS L. Interactions between synthetic vanilloids and the endogenous cannabinoid system. FEBS Lett. 1998;436:449–454. doi: 10.1016/s0014-5793(98)01175-2. [DOI] [PubMed] [Google Scholar]
- FEGLEY D., KATHURIA S., MERCIER R., LI C., GOUTOPOULOS A., MAKRIYANNIS A., PIOMELLI D. Anandamide transport is independent of fatty-acid amide hydrolase activity and is blocked by the hydrolysis-resistant inhibitor AM1172. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8756–8761. doi: 10.1073/pnas.0400997101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILLARD C.J., JARRAHIAN A.Cellular accumulation of anandamide: consensus and controversy Br. J. Pharmacol. 2003140802–808.Epub 2003 Sep 01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- HWANG S.W., CHO H., KWAK J., LEE S.Y., KANG C.J., JUNG J., CHO S., MIN K.H., SUH Y.G., KIM D., OH U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc. Natl. Acad. Sci. U.S.A. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORDT S.E., JULIUS D. Molecular basis for species-specific sensitivity to ‘hot' chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- JUNG J., HWANG S.W., KWAK J., LEE S.Y., KANG C.J., KIM W.B., KIM D., OH U. Capsaicin binds to the intracellular domain of the capsaicin-activated ion channel. J. Neurosci. 1999;19:529–538. doi: 10.1523/JNEUROSCI.19-02-00529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOLLARIK M., UNDEM B.J. Mechanisms of acid-induced activation of airway afferent nerve fibres in guinea-pig. J. Physiol. 2002;543:591–600. doi: 10.1113/jphysiol.2002.022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU L., LO Y., CHEN I., SIMON S.A. The responses of rat trigeminal ganglion neurons to capsaicin and two nonpungent vanilloid receptor agonists, olvanil and glyceryl nonamide. J. Neurosci. 1997;17:4101–4111. doi: 10.1523/JNEUROSCI.17-11-04101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACCARRONE M., BARI M., LORENZON T., BISOGNO T., DI MARZO V., FINAZZI-AGRO A. Anandamide uptake by human endothelial cells and its regulation by nitric oxide. J. Biol. Chem. 2000;275:13484–13492. doi: 10.1074/jbc.275.18.13484. [DOI] [PubMed] [Google Scholar]
- RICCIO M.M., KUMMER W., BIGLARI B., MYERS A.C., UNDEM B.J. Interganglionic segregation of distinct vagal afferent fibre phenotypes in guinea-pig airways. J. Physiol. 1996;496:521–530. doi: 10.1113/jphysiol.1996.sp021703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAVIDGE J., DAVIS C., SHAH K., COLLEY S., PHILLIPS E., RANASINGHE S., WINTER J., KOTSONIS P., RANG H., MCINTYRE P. Cloning and functional characterization of the guinea pig vanilloid receptor 1. Neuropharmacology. 2002;43:450–456. doi: 10.1016/s0028-3908(02)00122-3. [DOI] [PubMed] [Google Scholar]
- STEBBINS K.J., CARR M.J., PEDAPATI E.V., ELLIS J.L. Effect of olvanil on the afferent and efferent function of capsaicin-sensitive C-fibers in guinea pig airways. Eur. J. Pharmacol. 2003;471:205–211. doi: 10.1016/s0014-2999(03)01833-8. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- SZALLASI A., DI MARZO V. New perspectives on enigmatic vanilloid receptors. Trends Neurosci. 2000;23:491–497. doi: 10.1016/s0166-2236(00)01630-1. [DOI] [PubMed] [Google Scholar]
- TUCKER R.C., KAGAYA M., PAGE C.P., SPINA D. The endogenous cannabinoid agonist, anandamide stimulates sensory nerves in guinea-pig airways. Br. J. Pharmacol. 2001;132:1127–1135. doi: 10.1038/sj.bjp.0703906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNDEM B.J., CHUAYCHOO B., LEE M.G., WEINREICH D., MYERS A.C., KOLLARIK M. Subtypes of vagal afferent C-fibres in guinea-pig lungs. J. Physiol. 2004;556:905–917. doi: 10.1113/jphysiol.2003.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNDEM B.J., KOLLARIK M. Characterization of the vanilloid receptor 1 antagonist iodo-resiniferatoxin on the afferent and efferent function of vagal sensory C-fibers. J. Pharmacol. Exp. Ther. 2002;303:716–722. doi: 10.1124/jpet.102.039727. [DOI] [PubMed] [Google Scholar]
- VAN DER STELT M., DI MARZO V. Endovanilloids. Putative endogenous ligands of transient receptor potential vanilloid 1 channels. Eur. J. Biochem. 2004;271:1827–1834. doi: 10.1111/j.1432-1033.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H., SORGARD M., DI MARZO V., JULIUS D., HOGESTATT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]